Criegee Oxidation
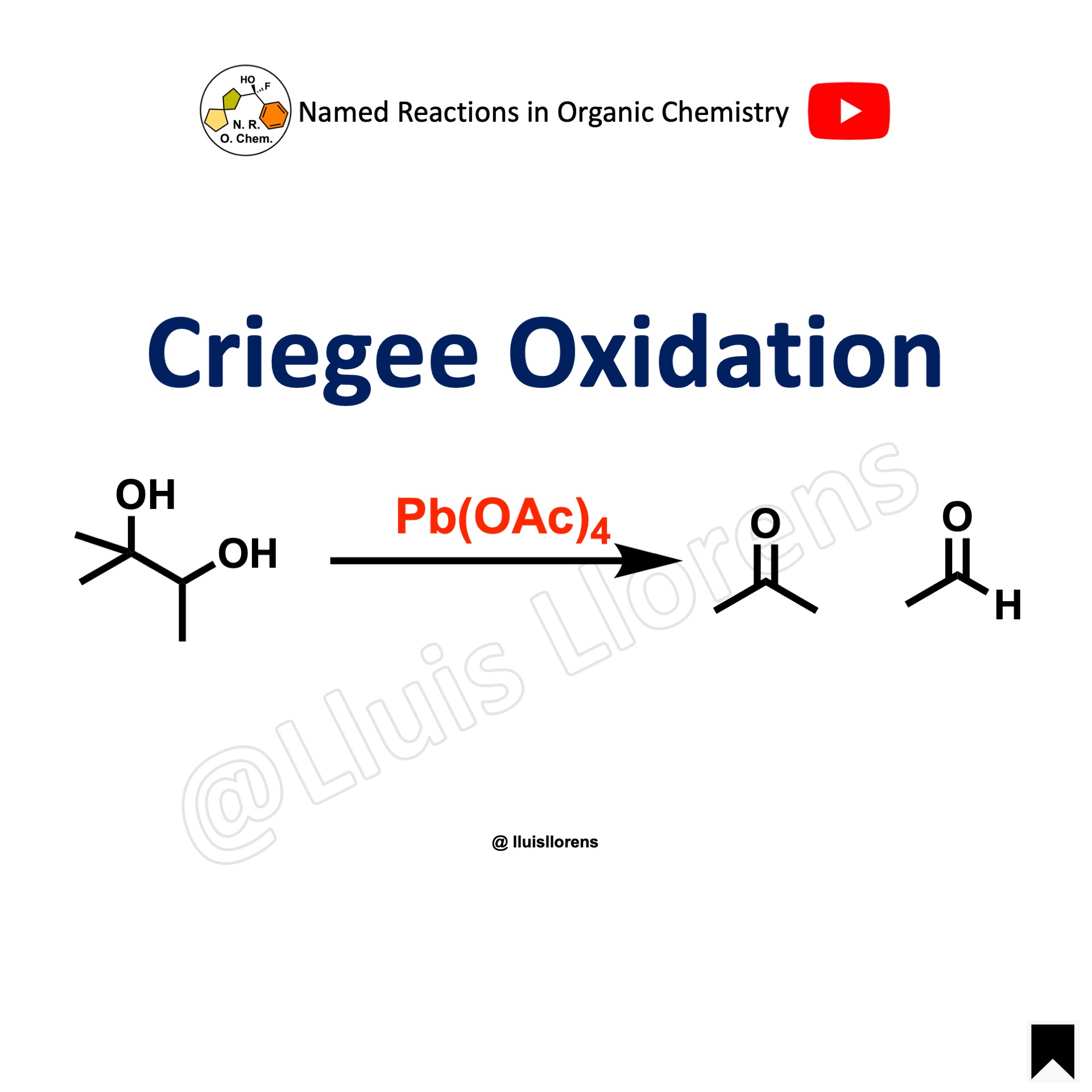
The Criegee oxidation is a 1,2-diol (glycol) cleavage reaction in which vicinal diols are oxidized to form ketones and aldehydes using lead tetraacetate. Several oxidizing agents such as HIO4, PIDA, cerium(IV) salts, or silver(I) salts also cleave glycols.
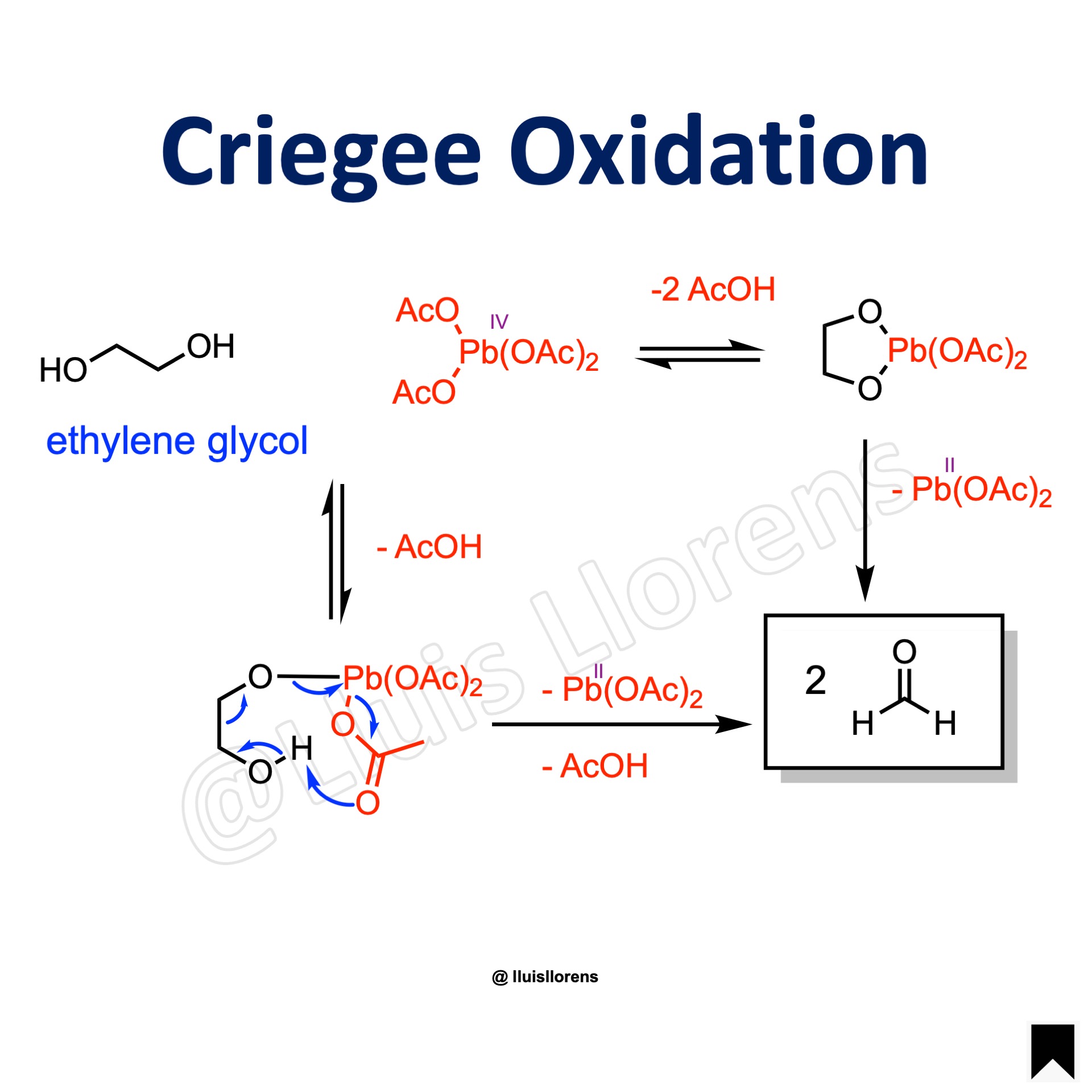
Reaction Mechanism
1. Formation of a bidentate metal, a 1,2-glycol five-membered complex which then breaks down to products via a two-electron process. 2. An alternative concerted electron displacement involving one of the acetate groups attached to the metal is also possible.
Experimental Procedure
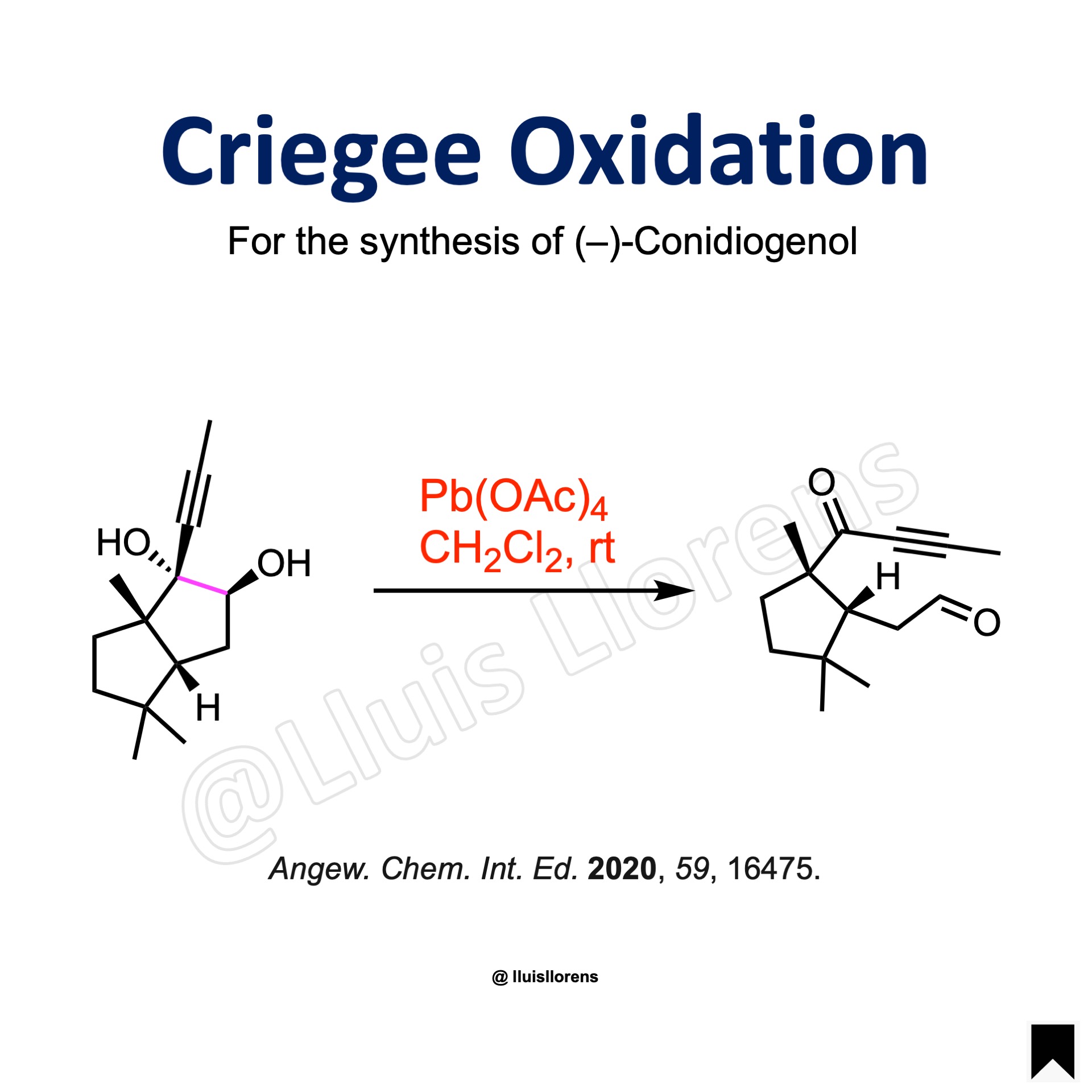
To a stirred suspension of Pb(OAc)4 (1.5 eq) in dichloromethane (25 mL), a solution of the 1,2-diol (2.25 mmol, 1.0 eq) in dichloromethane (10 mL) was added. The resulting mixture was stirred at room temperature for 20 min before quenched with ethylene glycol. Then, the volatiles were removed under reduced pressure to give the crude product that was used in the next step without further purification.
Learn More Named Reactions
[instagram-feed feed=2]