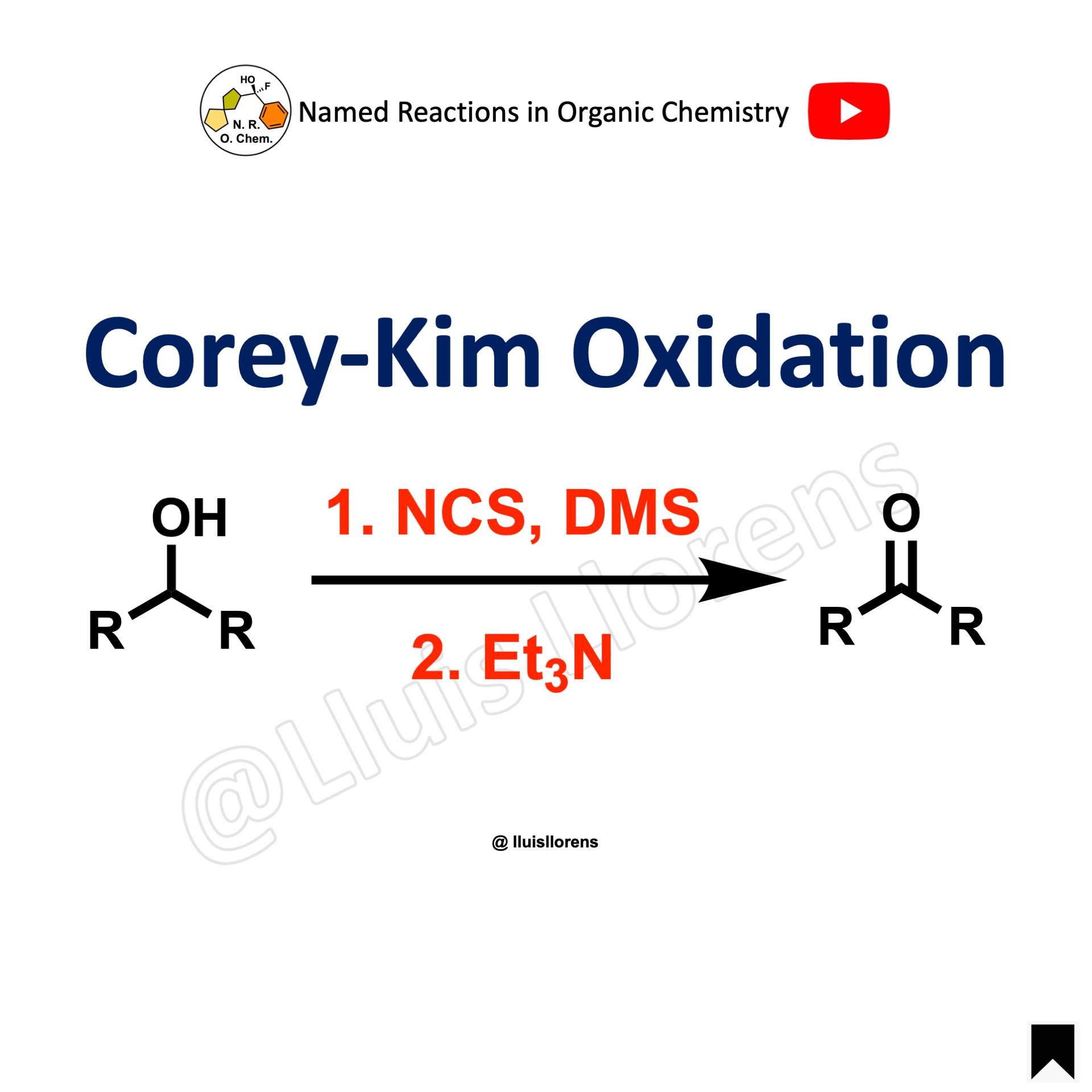
Corey-Kim Oxidation
The Corey-Kim oxidation is the synthesis of aldehydes and ketones from primary and secondary alcohols using N-chlorosuccinimide (NCS), dimethyl sulfide (DMS), and an organic base (Et3N).
General features:
1. The reaction conditions for the oxidation are mild and tolerate many functional groups. 2. The procedure is used to oxidize a wide range of alcohols except for allylic and benzylic alcohols. In which case the alcohols are converted to the allylic and benzylic halides. 3. To avoid the unpleasant odor of DMS, dodecylmethylsulfide is often used.
Reaction Mechanism
1. Reaction between dimethylsulfide and NCS generates a sulfonium salt. 2. The sulfonium salt is attacked by the nucleophilic alcohol to afford an alkoxysulfonium salt. 3. The alkoxysulfonium salt is deprotonated by triethylamine to deliver the corresponding carbonyl compound and dimethyl sulfide.
Experimental Procedure
To a stirred suspension of freshly recrystallized N-chlorosuccinimide (2.0 eq) in toluene (6.0 mL) at 0 °C was added dry dimethylsulfide (2.4 eq), and the resulting solution was cooled to –20 °C and stirred for 40 min. The alcohol (0.51 mmol, 1.0 eq) in toluene (5.0 mL) was added dropwise to the reaction mixture over 15 min. This was allowed to stir at –20 °C for 30 min and then at 0 ºC for 30 min. Triethylamine (4.0 eq) was then added. The reaction mixture was warmed to ambient temperature, stirred for 20 min, quenched with water and diethyl ether, and stirred vigorously for 5 min. The water layer was extracted with diethyl ether, and the organic phases were dried over sodium sulfate, filtered, and evaporated in vacuo to yield the crude product that was used without further purification in the next step.
Learn More Named Reactions
[instagram-feed feed=2]