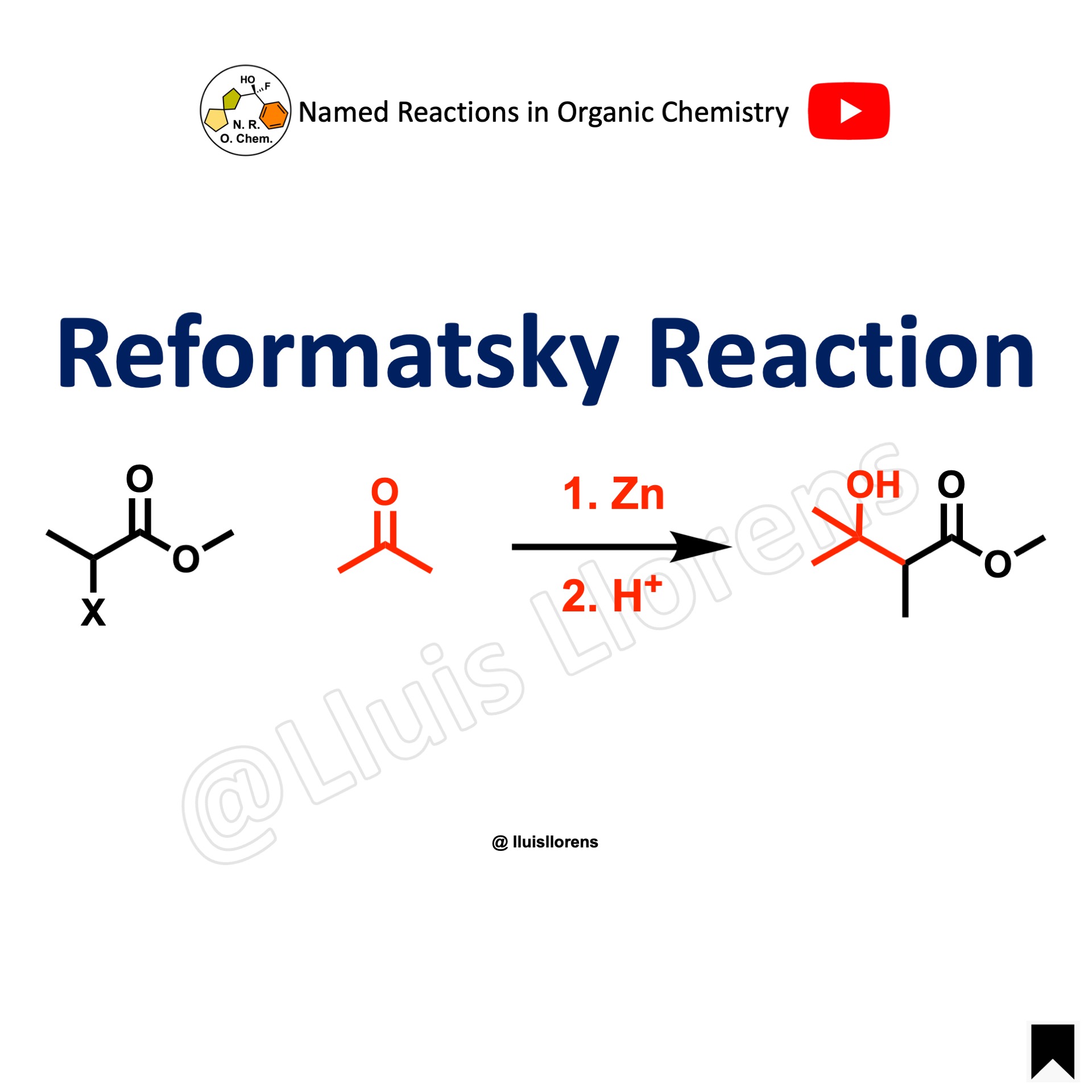
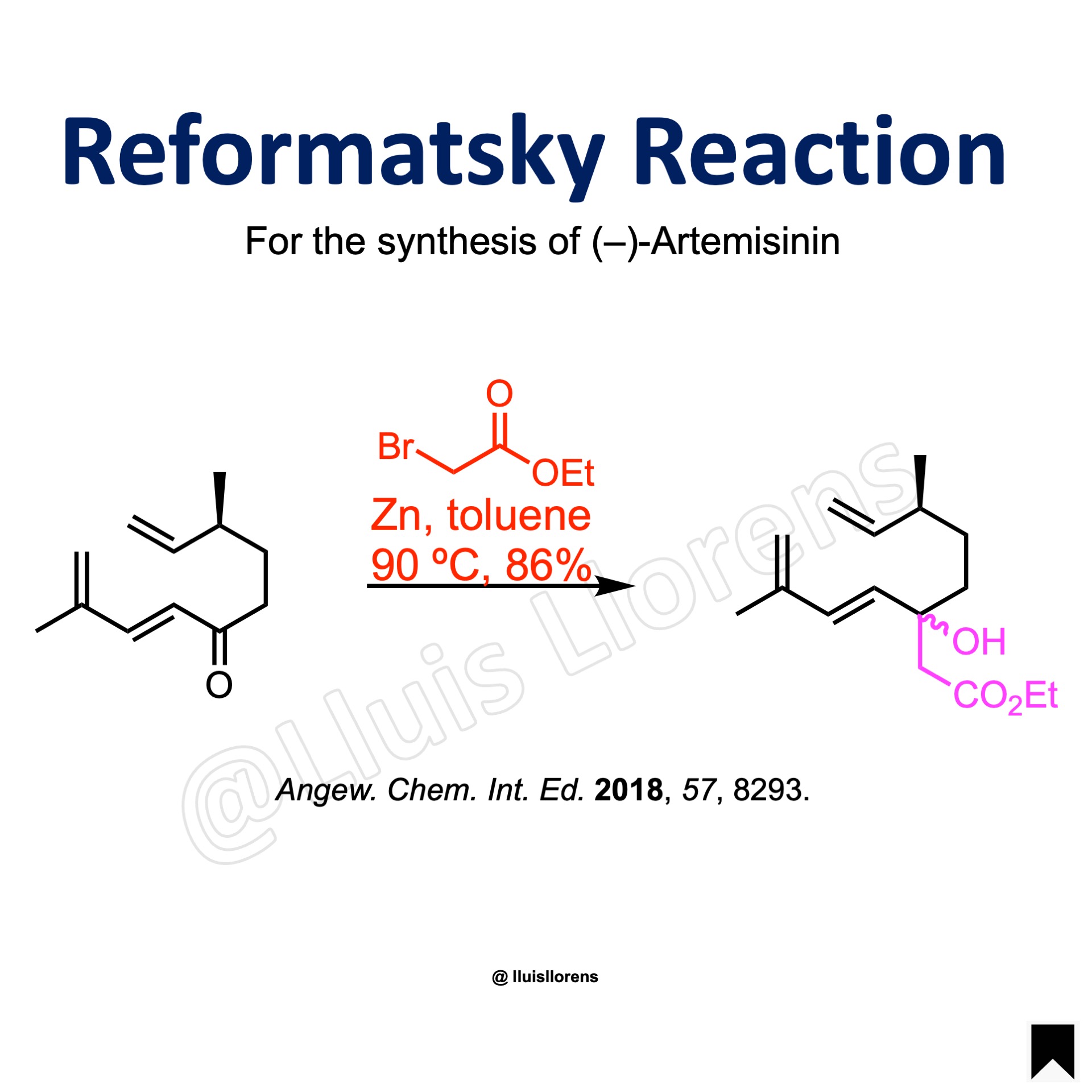
Reformatsky Reaction
The Reformatsky reaction is the zinc-induced reaction between alpha-halo esters and aldehydes or ketones. Also, a metal-induced reaction of alpha-carbonyl halides with a wide range of electrophiles.
General features:
1. The Reformatsky reaction is a good alternative to the classical aldol reaction. 2. The reaction works with highly substituted ketone substrates. 3. The organozinc reagent, or ‘Reformatsky enolate,’ is prepared by treating an alpha-halo ester with zinc dust. 4. The ester enolate can be formed in the presence of highly enolizable aldehyde and ketone functionalities. 5. The Reformatsky reaction is suitable for intramolecular reactions.
Reaction Mechanism
1. Oxidative addition. The activated zinc metal inserts into the carbon-halogen bond. 2. The resulting compound dimerizes and rearranges to form two zinc enolates. 3. Reaction of the zinc enolate with the carbonyl compound (aldol reaction) through a six-membered chair-like transition state. 4. Acid workup removes zinc to yield the corresponding β-hydroxy ester.
Experimental Procedure
A suspension of activated zinc dust (5.0 eq), iodine (0.1 eq) and toluene (50 mL) was stirred under reflux for 5 min and cooled to room temperature. To this mixture ethyl bromoacetate (2.0 eq) was added first. Afterwards, the ketone (5.61 mmol, 1.0 eq) solved in toluene (10 mL) was added to the suspension. The resulting mixture was stirred at 90 °C for 30 min. The reaction was cooled to 0 °C and water was added. The suspension was filtered, and the filtrate was extracted with MTBE. The combined organic phases were washed with water and brine, dried over Na2SO4, and concentrated under reduced pressure. The crude product was purified by silica gel chromatography to deliver the desired β-hydroxy ester (86% yield).
Learn More Named Reactions
[instagram-feed feed=2]