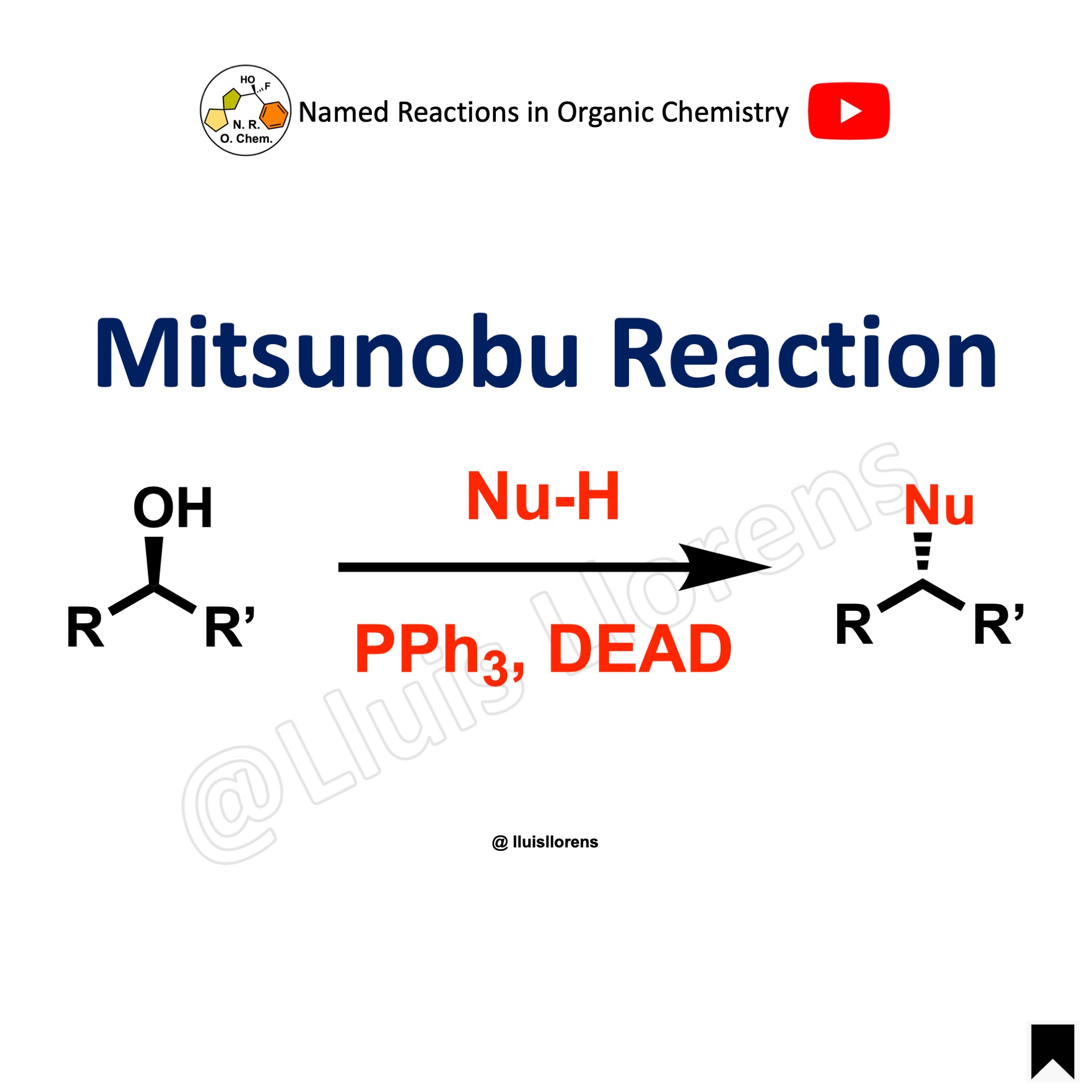
Mitsunobu Reaction
The Mitsunobu reaction allows the conversion of primary and secondary alcohols into different functional groups using triphenylphosphine and an azodicarboxylate. The reaction proceeds with inversion of configuration (SN2).
General features:
1. It is best suited for primary and secondary alcohols. Generally, tertiary alcohols don’t react. 2. Carboxylic acids afford esters; alcohols and phenols, ethers; thiols, thioethers. 3. The reaction is usually conducted in THF. Dioxane and DCM are also suitable. 4. PPh3 are the most common phosphines used. 5. DEAD and DIAD are the most common azodicarboxylate reagents used.
Reaction Mechanism
1. Triphenylphosphine reacts with DEAD producing a betaine intermediate that deprotonates the nucleophile. 2. Formation of the oxyphosphonium ion. 3. Nucleophilic attack of the nucleophile in an SN2 fashion generates the final product.
Experimental Procedure
To a solution of the alcohol (33.2 mmol, 1.0 eq) in THF (500 mL), 1,3-cyclopentanedione (2.42 eq) and triphenyl phosphine (2.50 eq) were added. The reaction mixture was stirred for 5 min at room temperature and then cooled to 0 °C. Diethyl azodicarboxylate solution (40% w.t. in toluene, 2.50 eq) was added at 0 °C over a period of 5 min, and the resultant brown solution was immediately placed in a pre-heated oil-bath (50 °C). The reaction mixture was stirred at 50 °C for 25 min and then cooled to room temperature. Removal of THF under reduced pressure afforded a black oil, which was diluted by Et2O and filtered over Celite. The filtrate was concentrated, and the residue was purified by column chromatography to give an orange solid-liquid mixture. Hexane was added, and the mixture was sonicated for 2 min and then filtered. The filtrate was concentrated under reduced pressure to give the desired product, which was directly used in the next step.
Learn More Named Reactions
[instagram-feed feed=2]