Named Molecules in Organic Chemistry
A post about reagents with interesting applications. Coined by the scientists who discovered them, chemists now harness their properties in their labs.
These molecules allow certain transformations that synthetic chemists have found useful in one way or another. Perhaps, they were used to achieve new and previously unprecedented transformations, or maybe they were just found superior to previously employed compounds.
Either way, these molecules present intriguing features worth looking at. And in this post, we are goning to review many of them. We will see their applications, how to prepare them or whether they are commercially available. We will talk about alternative procedures and compare them to other reagents. Oh, and please, make sure to come back because this post is going to keep growing.
Last update: 23/02/14
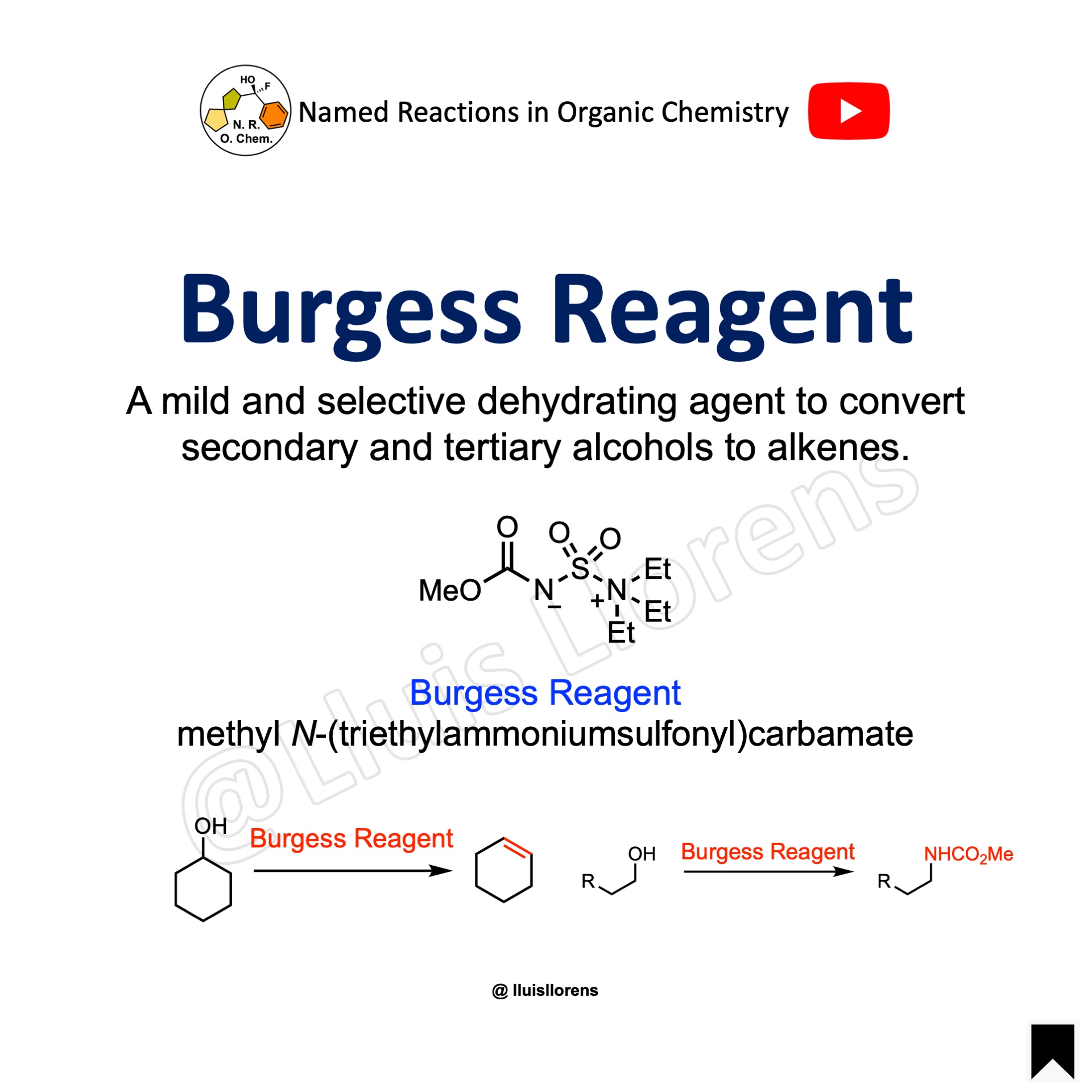
Burgess Reagent
A mild and selective dehydrating agent to convert secondary and tertiary alcohols with an adjacent proton to alkenes. The dehydration usually proceeds via a syn-elimination. Dehydration of primary alcohols does not work well with this reagent. In contrast to secondary and tertiary alcohols, primary alcohols lead to the formation of urethanes when exposed to the action of the Burgess reagent.
The reagent may also be used to oxidize primary and secondary alcohols to their corresponding aldehydes and ketones in dimethyl sulfoxide.
The Burgess reagent is commercially available but is easily prepared in two simple steps from chlorosulfonyl isocyanate by reaction between the isocyanate part and methanol to generate the carbamate, followed by reaction between the chlorosulfonyl group and triethylamine to give the sulfonamide part of the molecule. The white crystalline is air and moisture sensitive and, as such, needs to be stored in the cold and under an inert atmosphere.
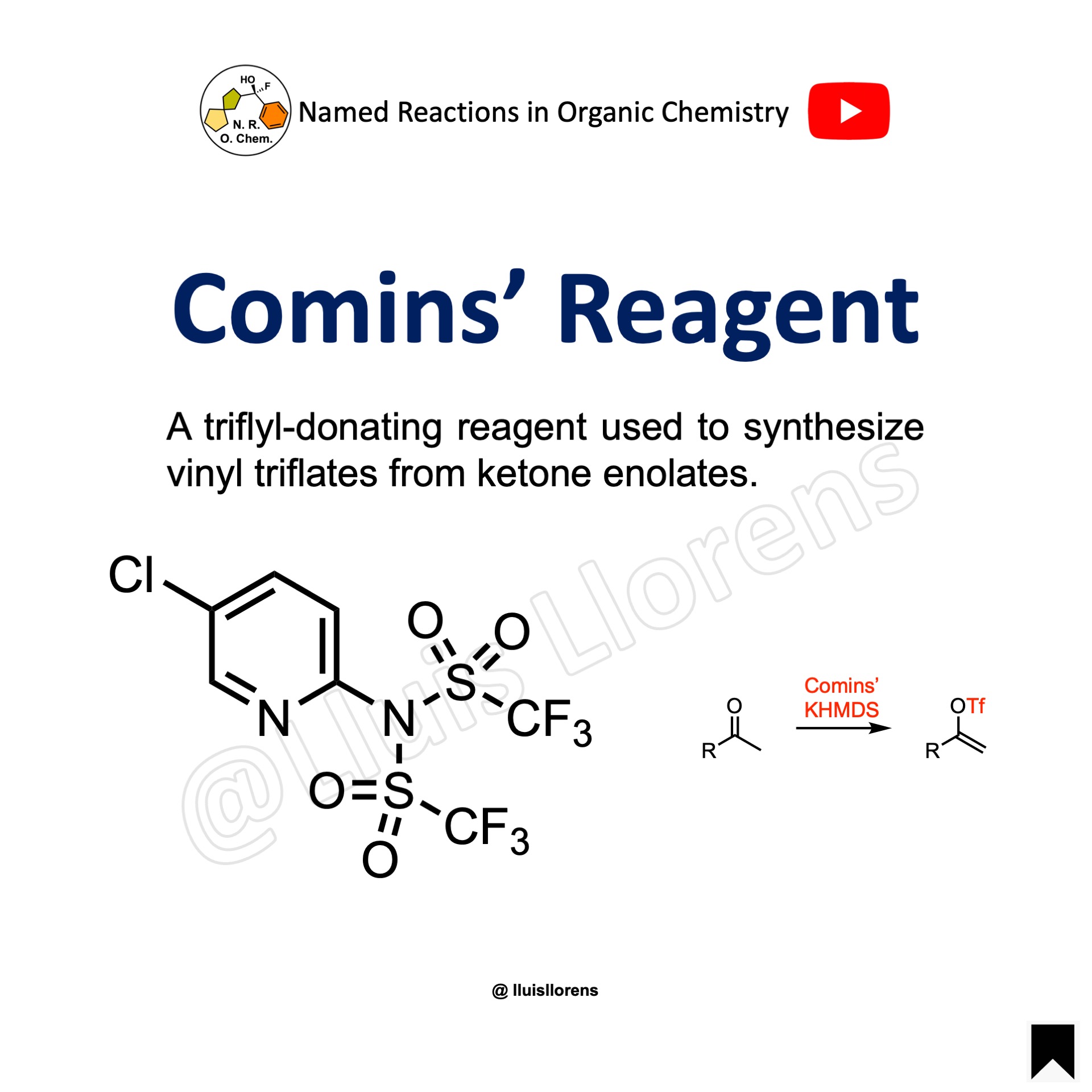
Comins’ Reagent
The Comins’ reagent is a triflyl-donating species that is used to synthesize vinyl triflates from the corresponding ketones. The vinyl triflates prepared are useful substrates for cross-coupling reactions.
It is more reactive than N-phenyltriflimide because the electron deficient aromatic ring of this pyridine-derived reagent withdraws electron density from the triflimide group causing it to be more susceptible to nucleophilic attack. In addition, there is also the possibility of activation through chelation by the pyridyl nitrogen to the metal of the metal enolate.
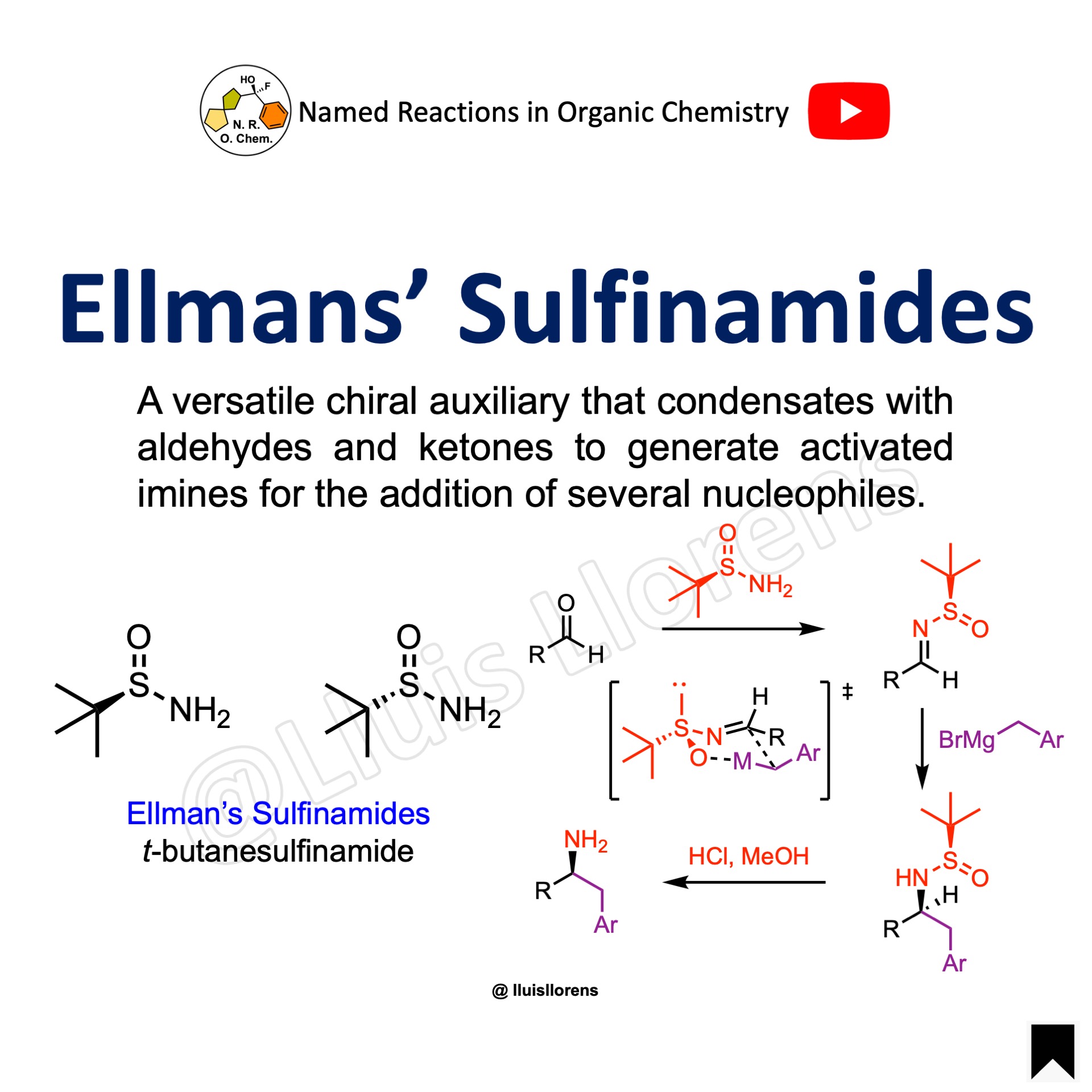
Ellman’s Sulfinamides
A chiral ammonia equivalent. It is a versatile chiral auxiliary that condensates with aldehydes and ketones to generate activated imines for the addition of several nucleophiles. It serves as a chiral directing group. The t-butanesulfonyl group is removed under mild conditions to provide the amine.
Sulfinamides, like sulfoxides with different substituents, contain sulfur atoms that are stereogenic centers (R1, R2, O atom, plus the lone pair: tetrahedral geometry with a trigonal pyramidal shape) and also configurationally stable. The preparation of enantioenriched sulfoxides is possible, making them optically active. The energy barrier required to invert this stereocenter is sufficiently high that sulfoxides are optically stable at room temperature.
Eschenmoser Salt
This reagent is a strong dimethylaminomethylating agent that may be used to convert ketones into enones.
The Eschenmoser salt is a useful reagent in organic synthesis. Named after Swiss chemist Albert Eschenmoser, this compound undergoes a Mannich type reaction with a ketone (e.g., enolate or silyl enol ether) to afford the corresponding tertiary amine. This amine can be further methylated to promote a base-induced elimination (Hofmann elimination). Or it may be oxidized to the corresponding amine oxide with an oxidant such as mCPBA to promote the Cope elimination.
Martins Sulfurane
Martin’s sulfurane is a ketal analog of diphenylsulfoxide with 2-phenyl-2-hexafluoropropanol prepared from the treatment of diphenyl sulfide with the potassium alkoxide form of the alcohol in carbon tetrachloride and stoichiometric amounts of bromine. The reagent is commercially available.
The Martin’s sulfurane is a very powerful dehydration reagent and can be applied to efficiently dehydrate alcohols under very mild conditions. One useful application for chemists is for the mild and selective dehydration of a secondary alcohol with an adjacent proton to give the corresponding alkene.
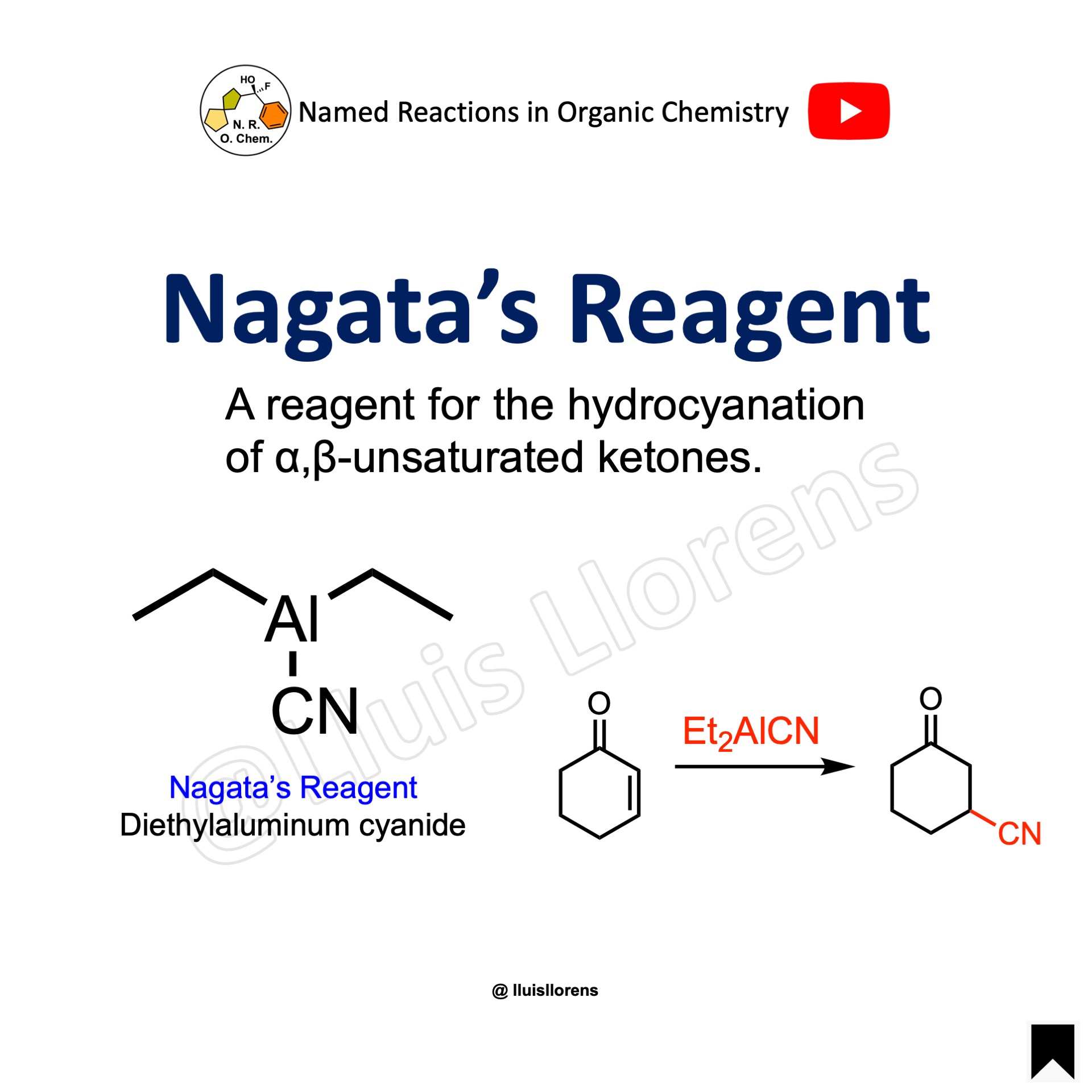
Nagata’s Reagent
Diethylaluminum cyanide is a useful, potent reagent for the hydrocyanation of various organic compounds. The Nagata reaction involves the conjugate hydrocyanation of an α,β-unsaturated carbonyl compound from diethylaluminum cyanide in an inert solvent. Ether, THF, and benzene are suitable solvents for the reaction.
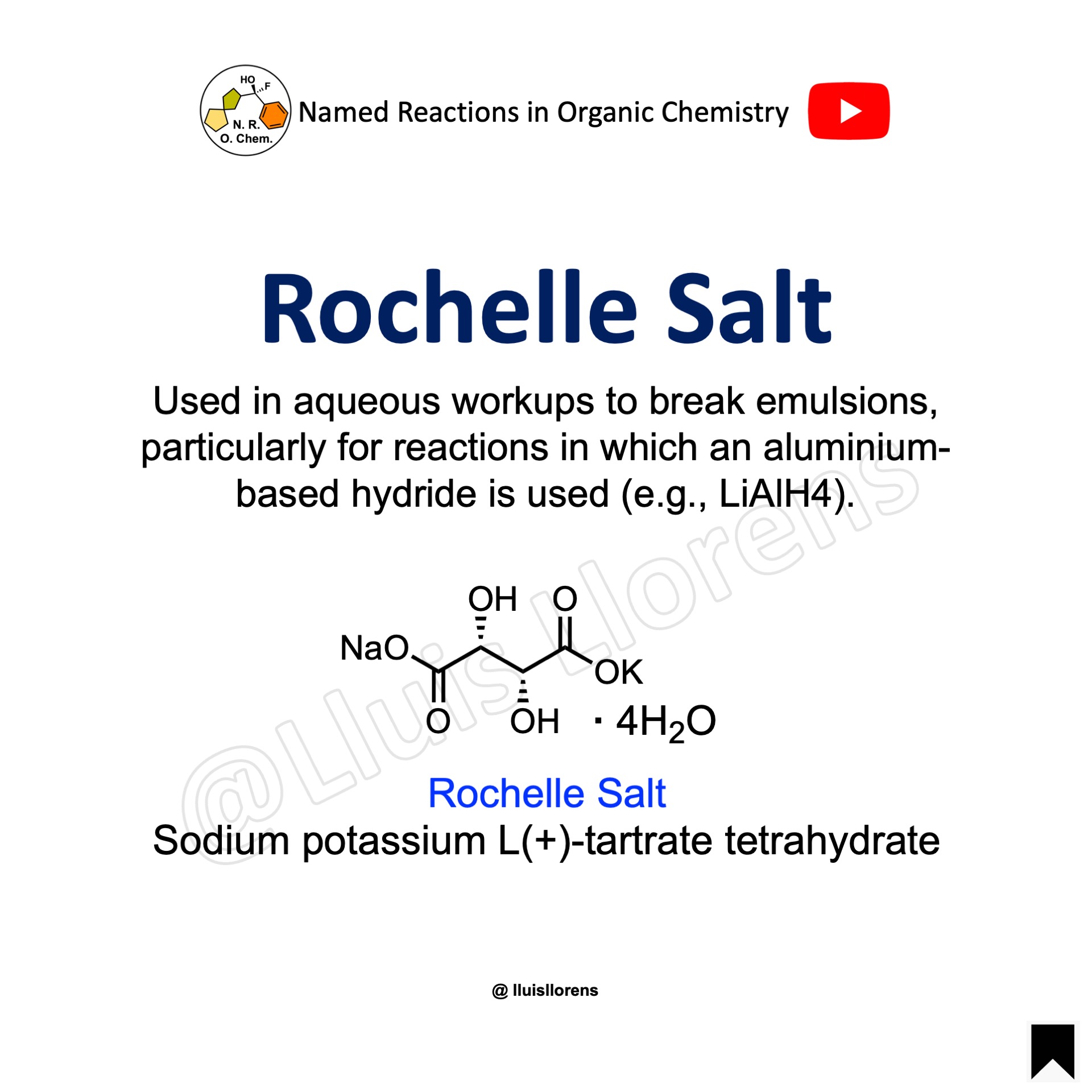
Rochelle Salt
The Rochelle salt is a type of sodium potassium tartrate mineral, which is found naturally in small crystal form. It has a wide range of uses, including as a food additive, in the pharmaceutical industry, and as a component in some types of batteries. It was first discovered in the town of La Rochelle in France in the 17th century by Pierre Seignette and has since become an important industrial mineral.
The molecule is best known as the Rochelle salt. Other names include the IUPAC name, sodium potassium L(+)-tartrate tetrahydrate, and Seignette salt, to honor the person who first reported its existence.
It is also known for its ability to expand and contract in response to temperature, which makes it useful in precision instruments. In the lab, it is used in aqueous workups to break emulsions, particularly for reactions in which an aluminum-based hydride is used (e.g., LiAlH4).
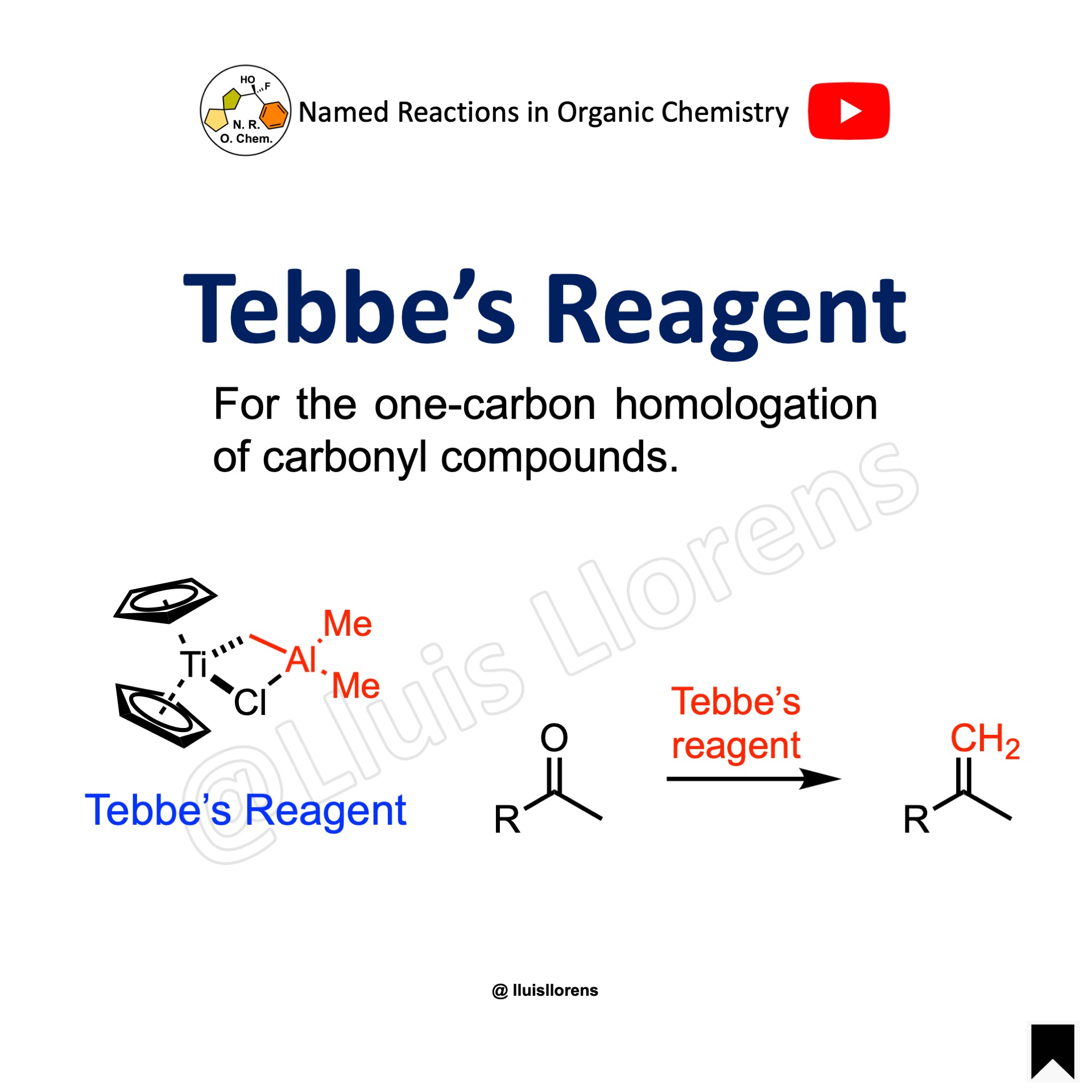
Tebbe’s Reagent
This reagent allows the one-carbon homologation of carbonyl compounds.
The Tebbe reagent is commercially available, but it can be prepared from the reaction between titanocene dichloride with two equivalents of trimethylaluminum in toluene. This generates a methylene-bridged titanium-aluminum complexed that is often referred to as the Tebbe reagent. The Tebbe reagent is more nucleophile and much less basic than the corresponding Wittig reagents. Therefore, less reactive carbonyl compounds can be readily olefinated, and it also presents more functional group tolerance.




Can you subscribe me to your portal?
We still don’t have any subscription plan nor newsletters, but it may change in the near future. For now, you are eligible to comment on the posts. Thank you for your interest.