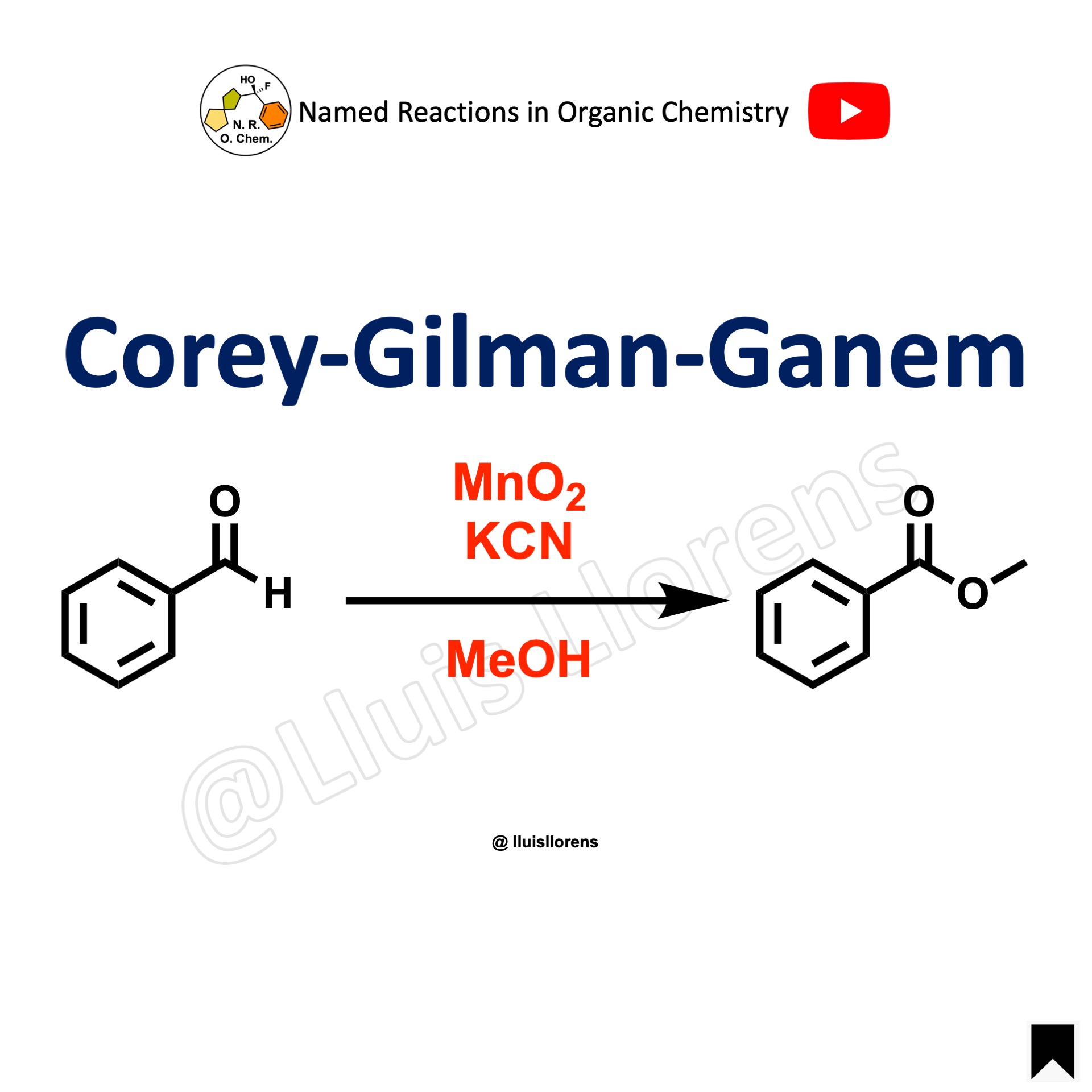
Corey-Gilman-Ganem Oxidation
The Corey-Gilman-Ganem oxidation allows the transformation of benzylic or α,β-unsaturated aldehydes into the corresponding methyl esters. The targeted aldehydes are oxidized to esters in the presence of MnO2 and potassium cyanide in methanol.
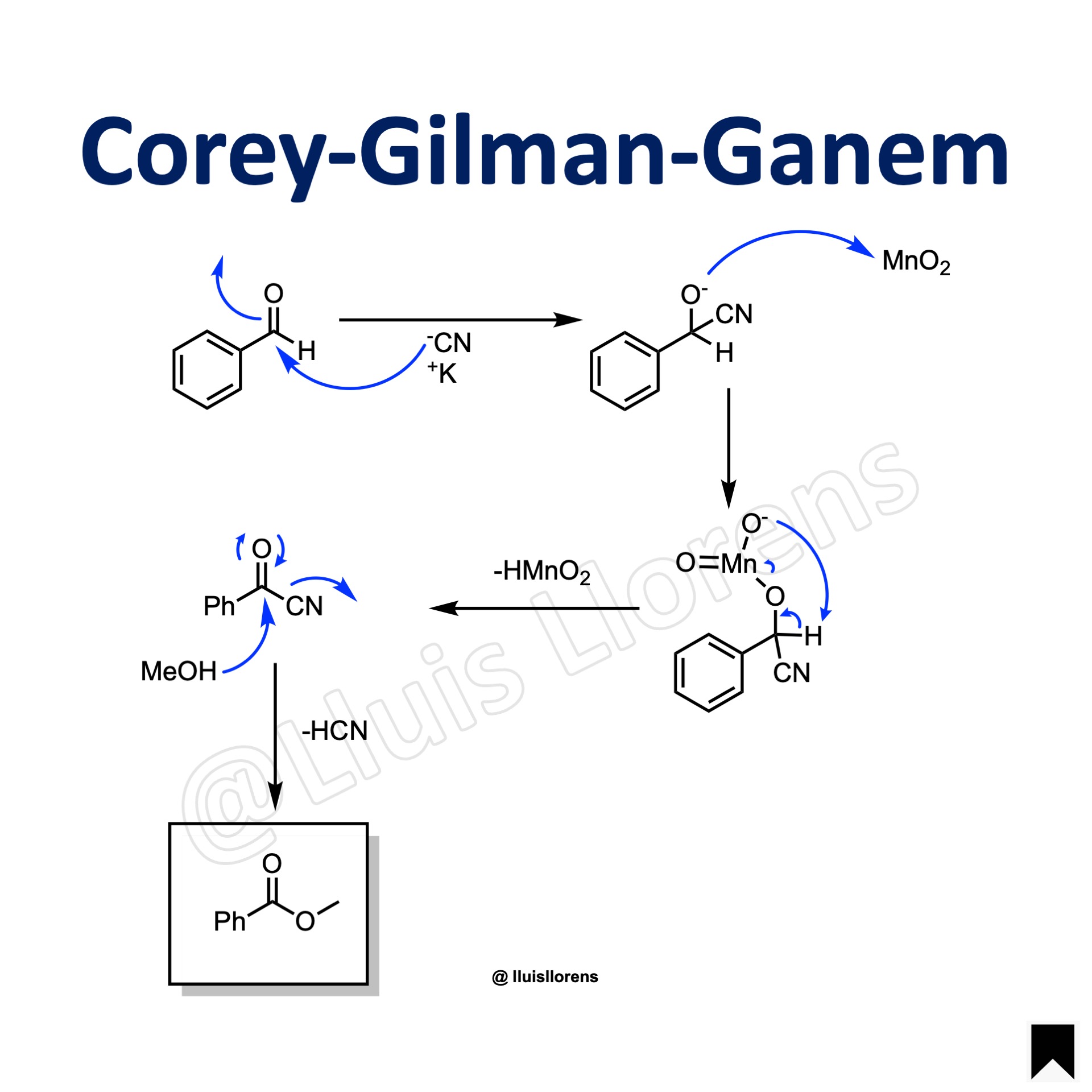
Reaction Mechanism
1. Nucleophilic attack by the cyanide ion to the carbonyl carbon of the aldehyde. 2. Addition of manganese dioxide to the cyanohydrin. 3. Pericyclic rearrangement results in the formation of acyl cyanide. 4. Addition of the alcohol and release of hydrogen cyanide lead to the resulting ester.
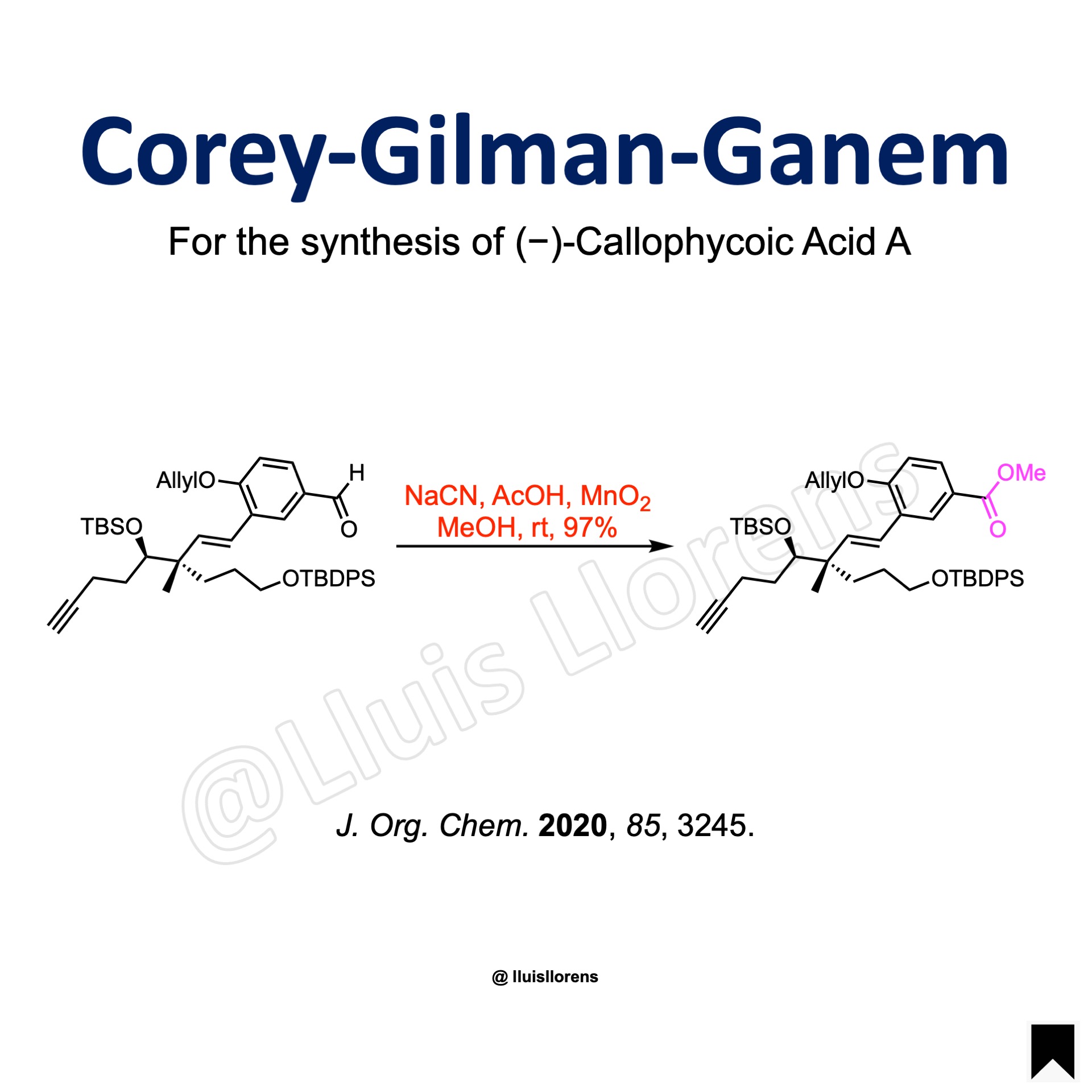
Example
Experimental Procedure
To a stirred solution of the aldehyde (1.26 mmol, 1.0 eq) in MeOH (15 mL) were added NaCN (4.85 eq), AcOH (1.35 eq) and MnO2 (17.8 eq). The mixture was stirred at room temperature for 16 h. The precipitated solids were removed by filtration through a pad of Celite and washed well with dichloromethane. The combined filtrate and washings were concentrated under reduced pressure. The residue was diluted with water and extracted with EtOAc. The combined extracts were dried and concentrated under reduced pressure. The residue was purified by column chromatography on silica gel to provide the desired methyl ester (97% yield).
New Release Out Now!
The Chemists' Cookbook
The Chemists’ Cookbook will be your favorite study or lab companion. It features 201 named reactions with detailed reaction schemes and experimental procedures. It includes a list of reactions organized by categories, making it simple to find reactions that perform similar transformations or target specific functional groups. Available as an ebook or in paperback.
NEW RELEASE OUT NOW!
The Chemists' Cookbook
The Chemists’ Cookbook will be your favorite study or lab companion. It features 201 named reactions with detailed reaction schemes and experimental procedures. It includes a list of reactions organized by categories, making it simple to find reactions that perform similar transformations or target specific functional groups. Available as an ebook or in paperback.