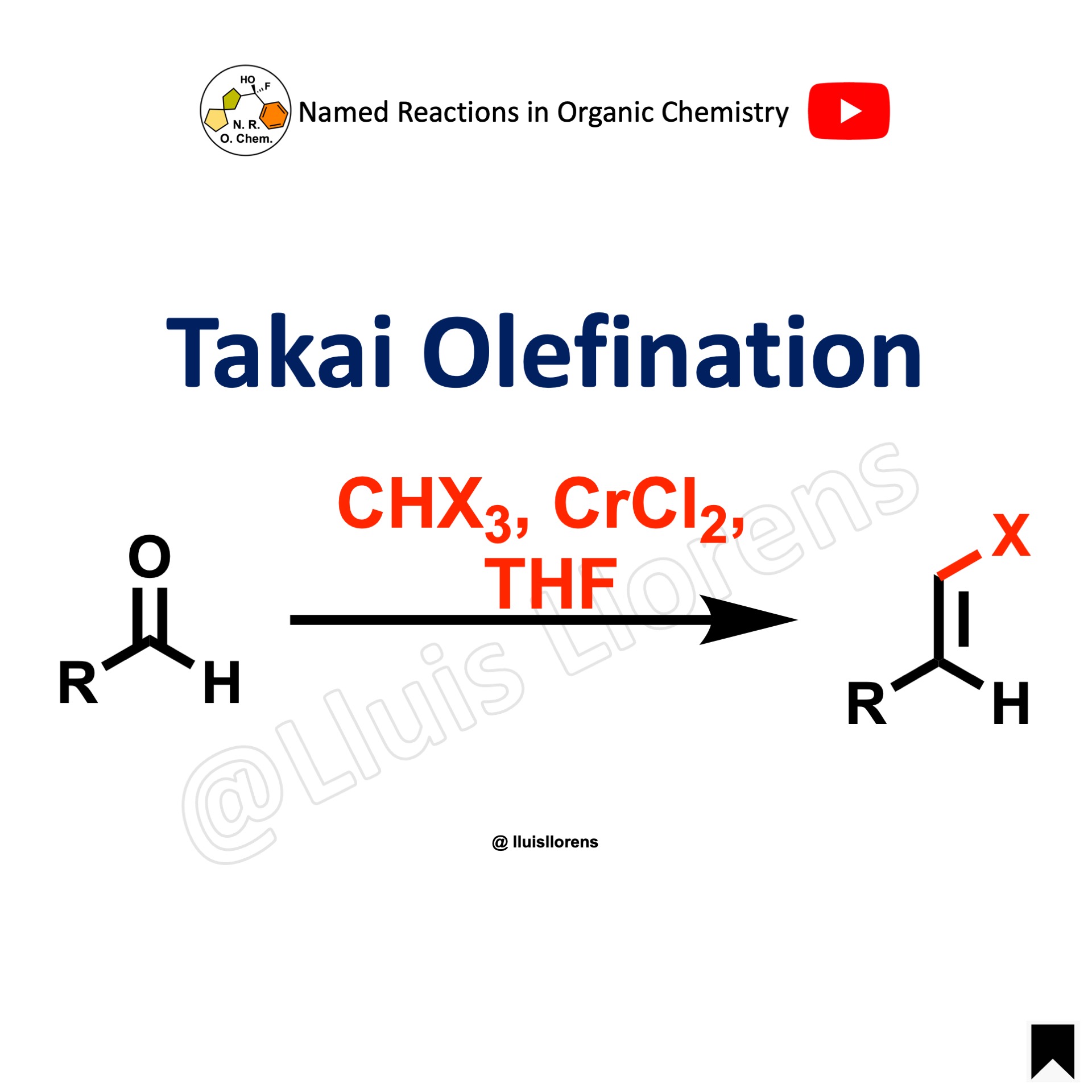
Takai Olefination
The Takai olefination is the chromium(II)-mediated one-carbon homologation of aldehydes with haloform.
General features:
1. The haloform used can be iodoform, bromoform, or chloroform, being iodoform the most reactive and chloroform the one that gives the best E-selectivity. 2. One main advantage of this reaction is the E-configuration of the double bond that is formed. However, the reaction requires the use of at least 4 equivalents of chromium chloride.
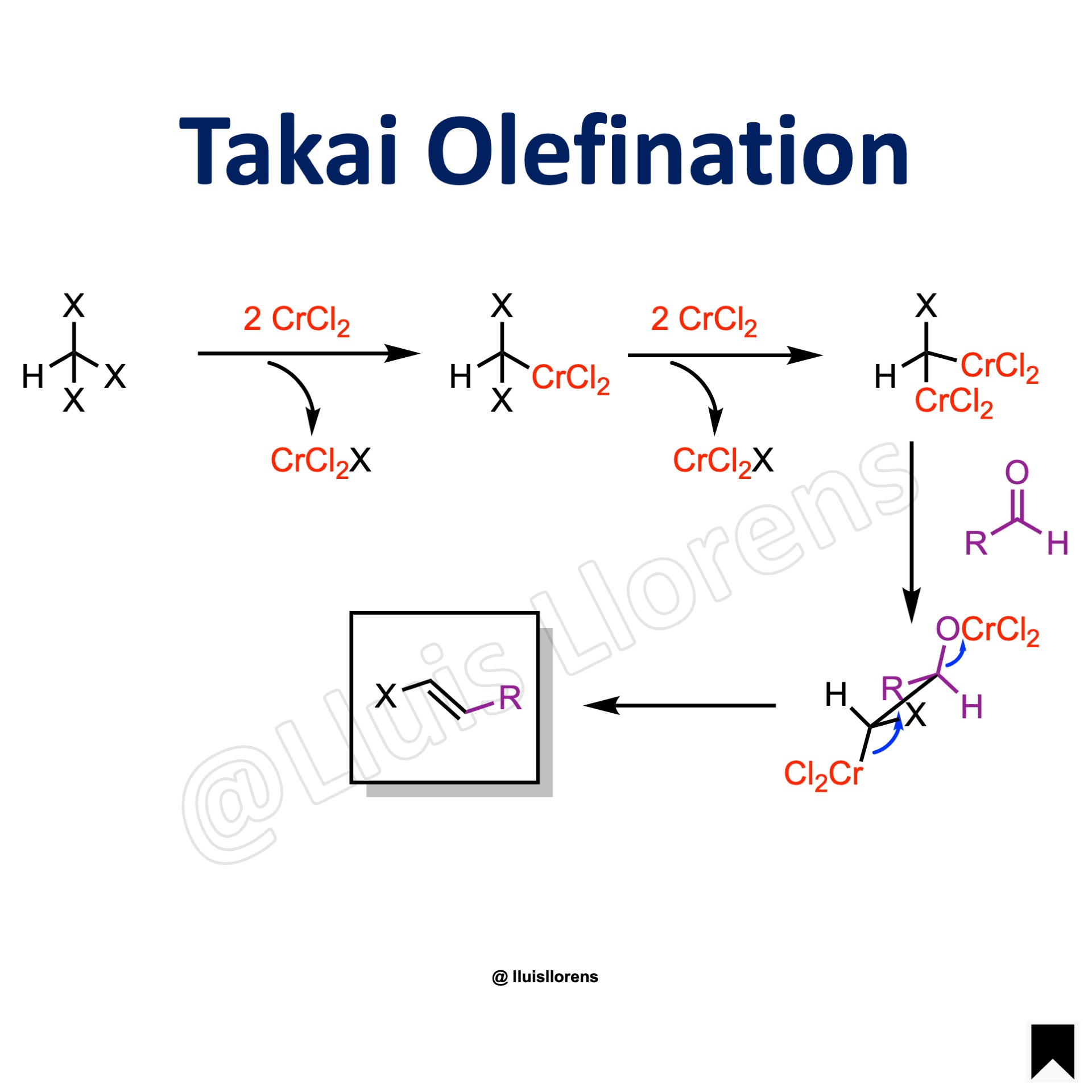
Reaction Mechanism
1. The haloform reacts with two equivalents of chromium chloride, and the chromium (II) species is oxidized to chromium (III). 2. This generates a geminal dichromium species that is nucleophilic and attacks the aldehyde in a 1,2-addition. 3. Finally, both chromium bearing groups engage in an elimination step.
Experimental Procedure
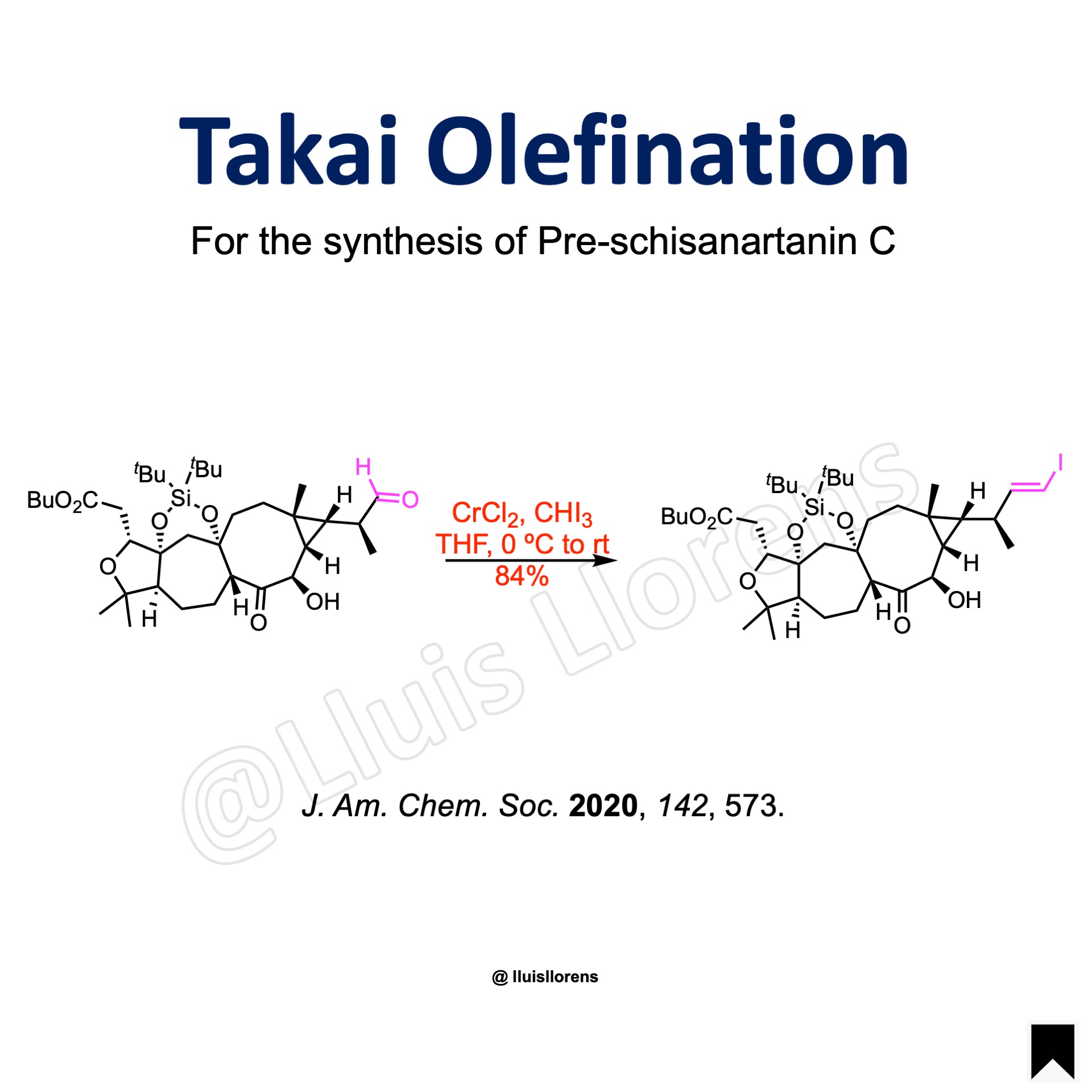
To a solution of CrCl2 (15.0 eq) in THF (1 mL) was added CHI3 (5.0 eq) at 0 ºC under N2, and the mixture was stirred at the same temperature for 15 min. To this solution was added a solution of the aldehyde (0.020 mmol, 1.0 eq) in THF (1 mL) at 0 ºC, the mixture was stirred at the same temperature for 1 h, and then at room temperature for 2 h. The reaction mixture was quenched by sequential addition of saturated solutions of NaHCO3, Na2EDTA, NaHSO3, and 2,6-di-tert-butylpyridine. The organic phase was separated, and the water phase was extracted with EtOAc. The combined organic phases were filtered off through a short silica gel pad, and washed with EtOAc. The filtrate was concentrated under vacuum, and the residue purified by flash column chromatography on silica gel to deliver the desired alkene (84% yield).
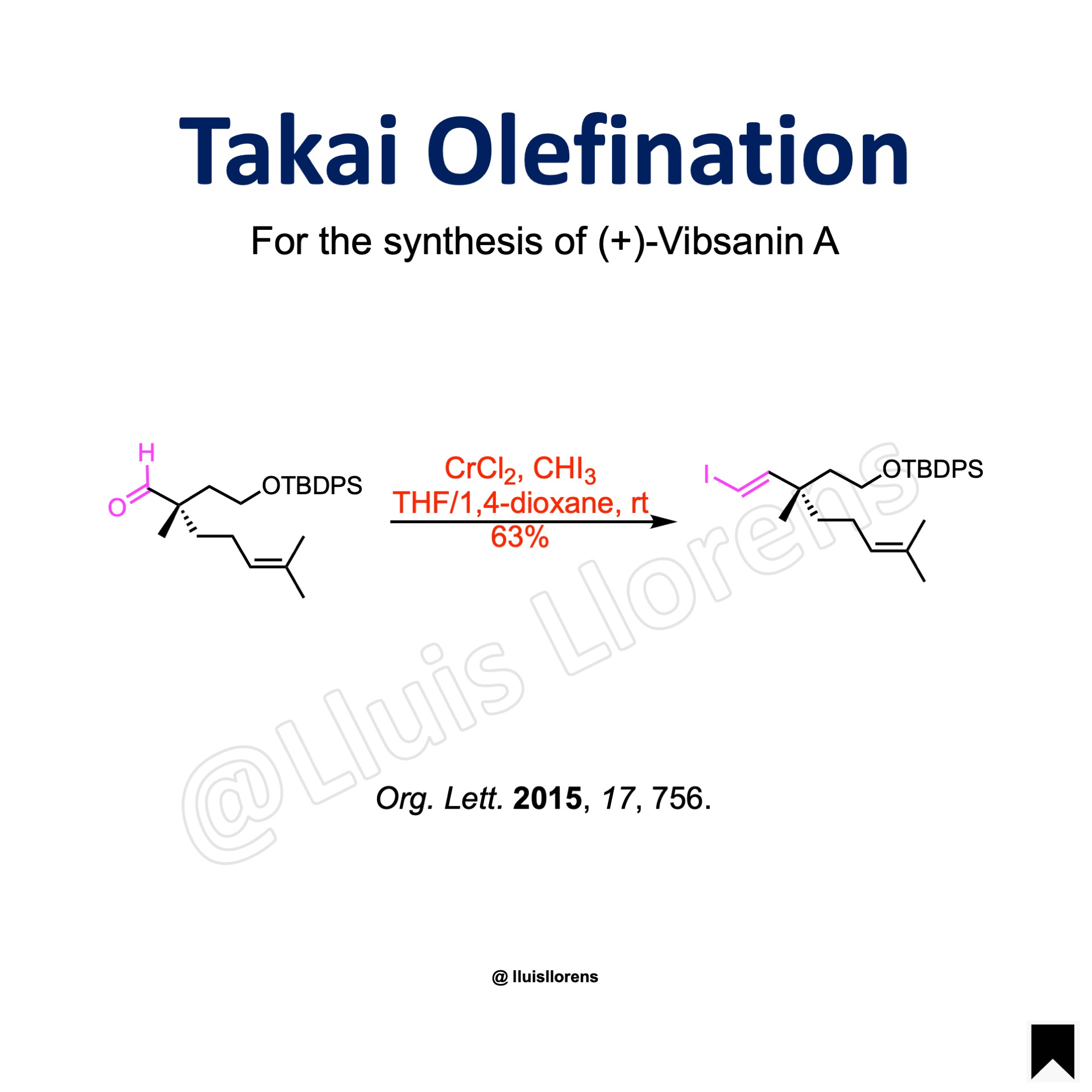
More Examples
Learn More Named Reactions
[instagram-feed feed=2]