Sandmeyer Reaction
The Sandmeyer reaction is the substitution of aryldiazonium salts with halides or pseudohalides.
General features:
1. The substrate is usually prepared from arylamines via diazotization. 2. Aryldiazonium halides are not isolated. Instead, they react in the same pot with copper(I) chloride, bromide, or cyanide to deliver the corresponding product. 3. The formation of aryl iodides can be carried out by adding potassium iodide. It does not require the use of a copper(I) salts. 4. Both electron-donating and electron-withdrawing groups are tolerated in the aromatic ring.
Reaction Mechanism
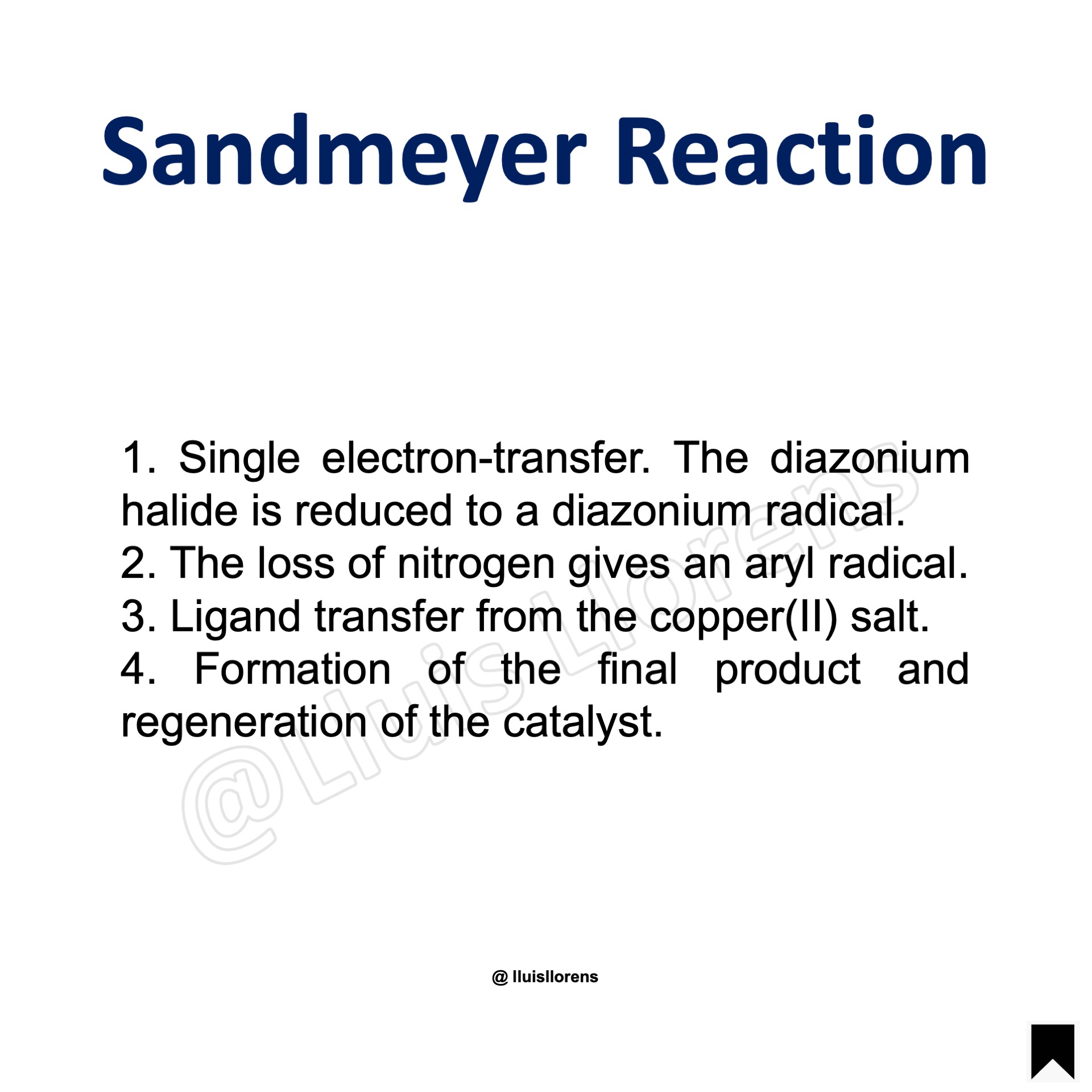
1. Single electron-transfer. The diazonium halide is reduced to a diazonium radical. 2. The loss of nitrogen gives an aryl radical. 3. Ligand transfer from the copper(II) salt. 4. Formation of the final product and regeneration of the catalyst.
Example
Experimental Procedure
To a stirred solution of the arylamine (3.0 mmol, 1.0 equiv) in deionized water (6.0 mL) was added concentrated sulfuric acid (2.8 equiv). The reaction mixture was cooled with an ice-salt bath. Then a solution of NaNO2 (1.2 equiv) in deionized water (1.5 mL) was added dropwise, and the reaction was stirred for 30 min. Then Et2O (3.0 mL) was added before NaI (4.0 equiv) in deionized water (1.5 mL) was added dropwise. The resulting mixture was warmed to room temperature and stirred for 3.0 h. Then saturated Na2S2O3 solution was added, and the mixture was extracted with EtOAc, and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography on silica gel to give the desired product (70% yield).
Learn More Named Reactions