Diels-Alder Reaction
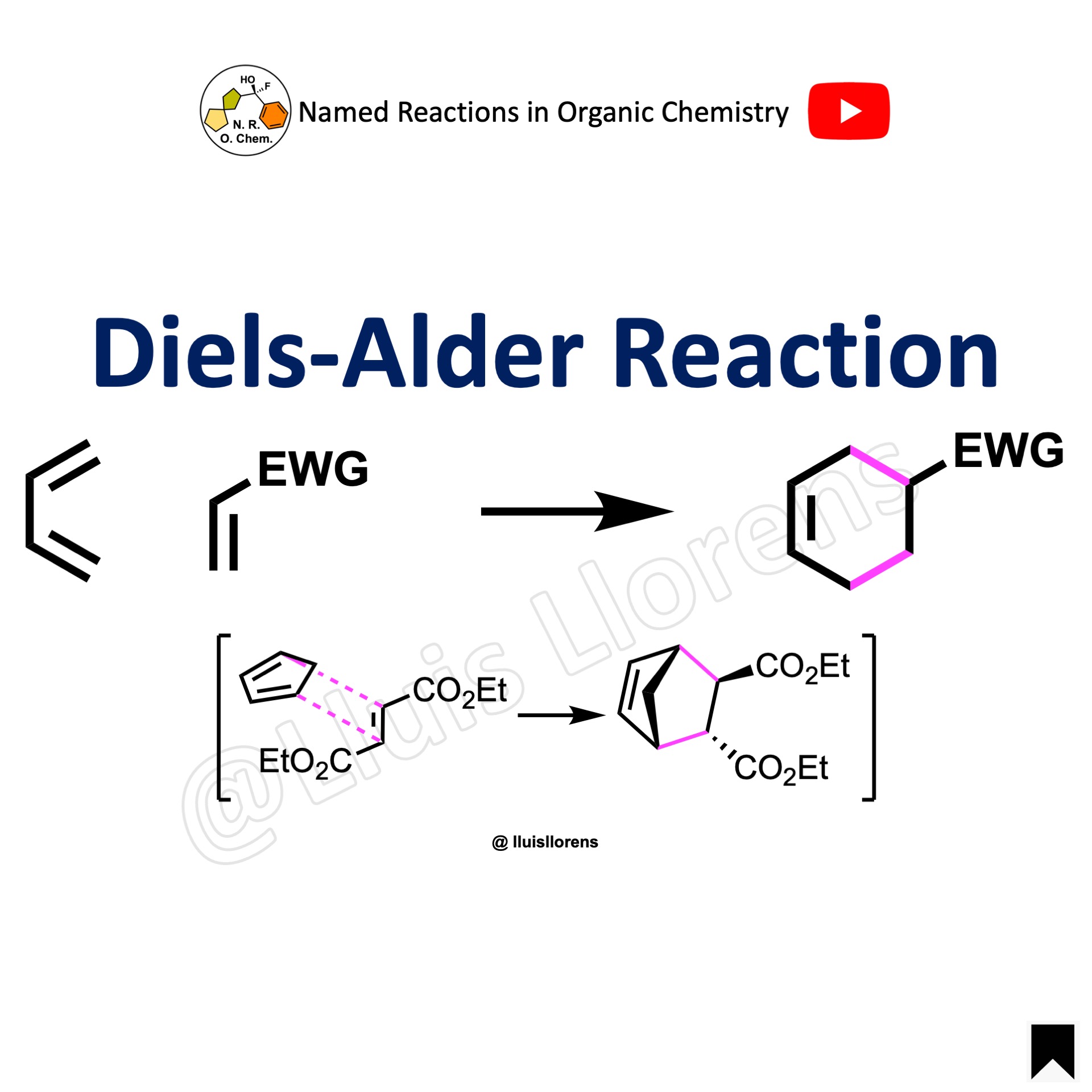
The Diels–Alder reaction is the [4 + 2] cyclization of a diene and a dienophile (i.e., an alkene) to generate a cyclohexene derivative.
The D-A reaction is a concerted, pericyclic reaction that generates two new σ-bonds through a cyclic transition state where the HOMO of the electron-rich component interacts with the LUMO of the electron-poor one. Usually, the diene component is electron-rich and the dienophile is electron-poor. But when the diene is electron-poor, by the presence of an electron-withdrawing group, and the dienophile electron-rich, by the presence of an electron-donating group, then the reaction is referred to as an inverse electron demand D-A cyclization.
General features:
1. The reaction is stereospecific, the stereochemical information of the diene and the dienophile (alkene) are retained in the product because the mechanism of the reaction demands this stereochemical outcome, just like in an SN2 reaction that goes with inversion of configuration. When a disubstituted (E) alkene is used, the stereochemistry of the two substituents in the product will be anti, but when a (Z) alkene is used instead, the stereochemistry in the product will be syn. Similarly, the stereochemical information (E or Z) in the diene is also transferred to the product. 2. The reaction is also highly regioselective and follows the so-called ortho-para rule. The regioisomers are predominantly the 1,2- and the 1,4-products over the 1,3-product. 3. The reaction is also stereoselective. In a stereoselective reaction, the molecule selects a particular reaction pathway for some reasons (be it steric hindrance, kinetics, thermodynamics, etc.) but the molecule isn’t forced by the mechanism to react one particular way. In this case, according to secondary orbital interactions, the predominant product is the endo cycloadduct. That is the product generated from the alkene that approaches the diene with the substituent towards the pi system. 4. If there is nothing impeding the olefin to access the diene from below or from above (e.g., by the presence of a chiral center), the reaction will give us a racemic product, that is a mixture of enantiomers.
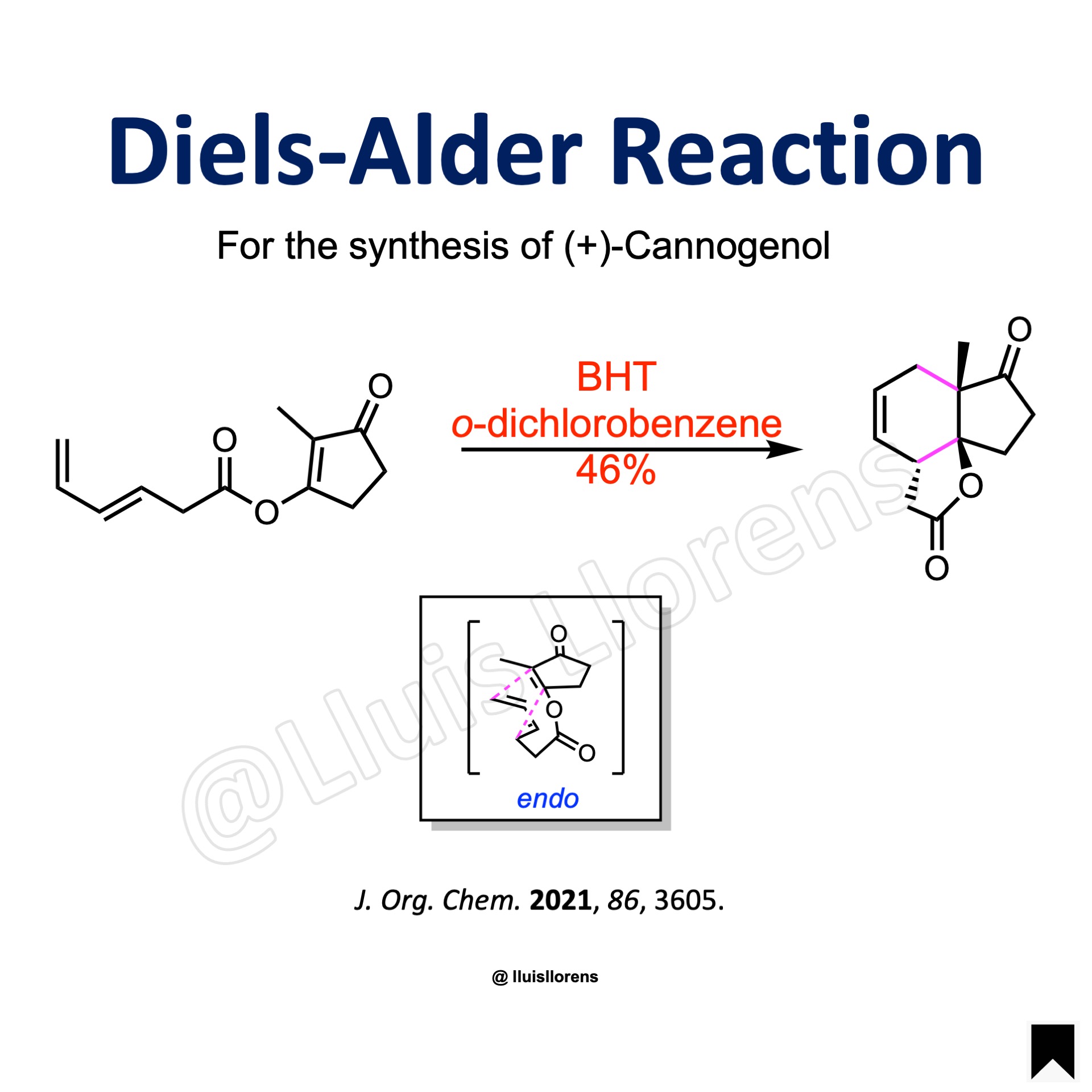
Example
Experimental Procedure
To a hot o-dichlorobenzene (1.6 L) were added BHT (16.0 mmol) and the substrate (400 mmol) in o-dichlorobenzene (500 mL) at 130 °C. The reaction mixture was heated to reflux and stirred for 6 h. The reaction mixture was concentrated under reduced pressure, and the residue was filtered through a pad of Celite. The filtrate was concentrated under reduced pressure, and the mixture was dissolved in toluene and charged onto a short pad of silica gel. The remaining o-dichlorobenzene was washed out by elution of hexane, and the remaining compounds were eluted with a mixture of CH2Cl2/Et2O. The resulting filtrate was concentrated under reduced pressure to afford a mixture of the cycloadducts ((±)-endo/(±)-exo = 81:19), which were separated and further purified by recrystallization and silica gel flash column chromatography.
More Examples
Learn More Named Reactions