Corey-Fuchs Homologation
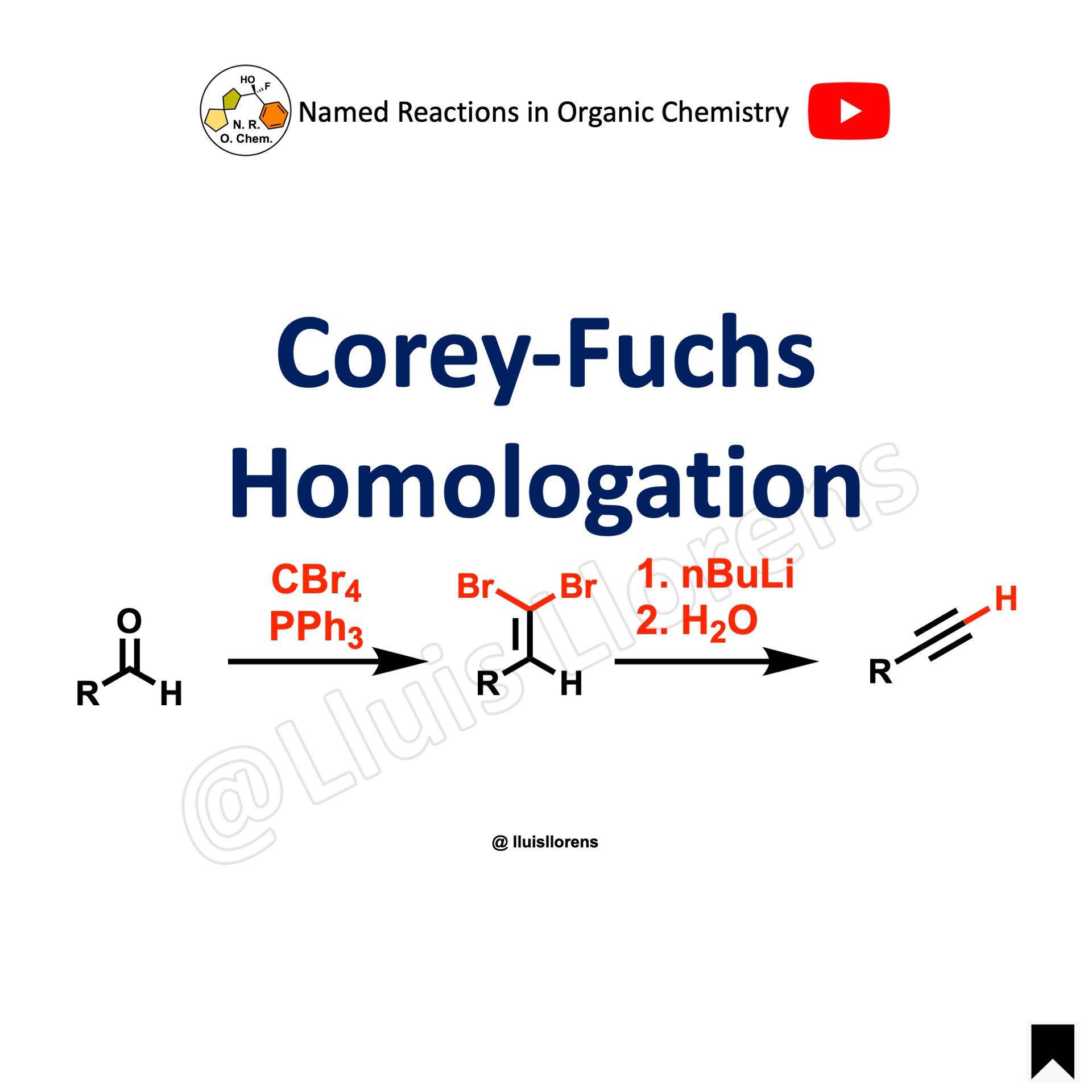
The Corey-Fuchs reaction is the one-carbon homologation of aldehydes to the corresponding terminal alkynes using carbon tetrabromide and triphenylphosphine.
General features:
1. For the transformation of the dibromoolefins to the terminal alkynes, the addition of 2 equivalents of n-butyllithium at -78 °C is needed. Then, hydrolysis.
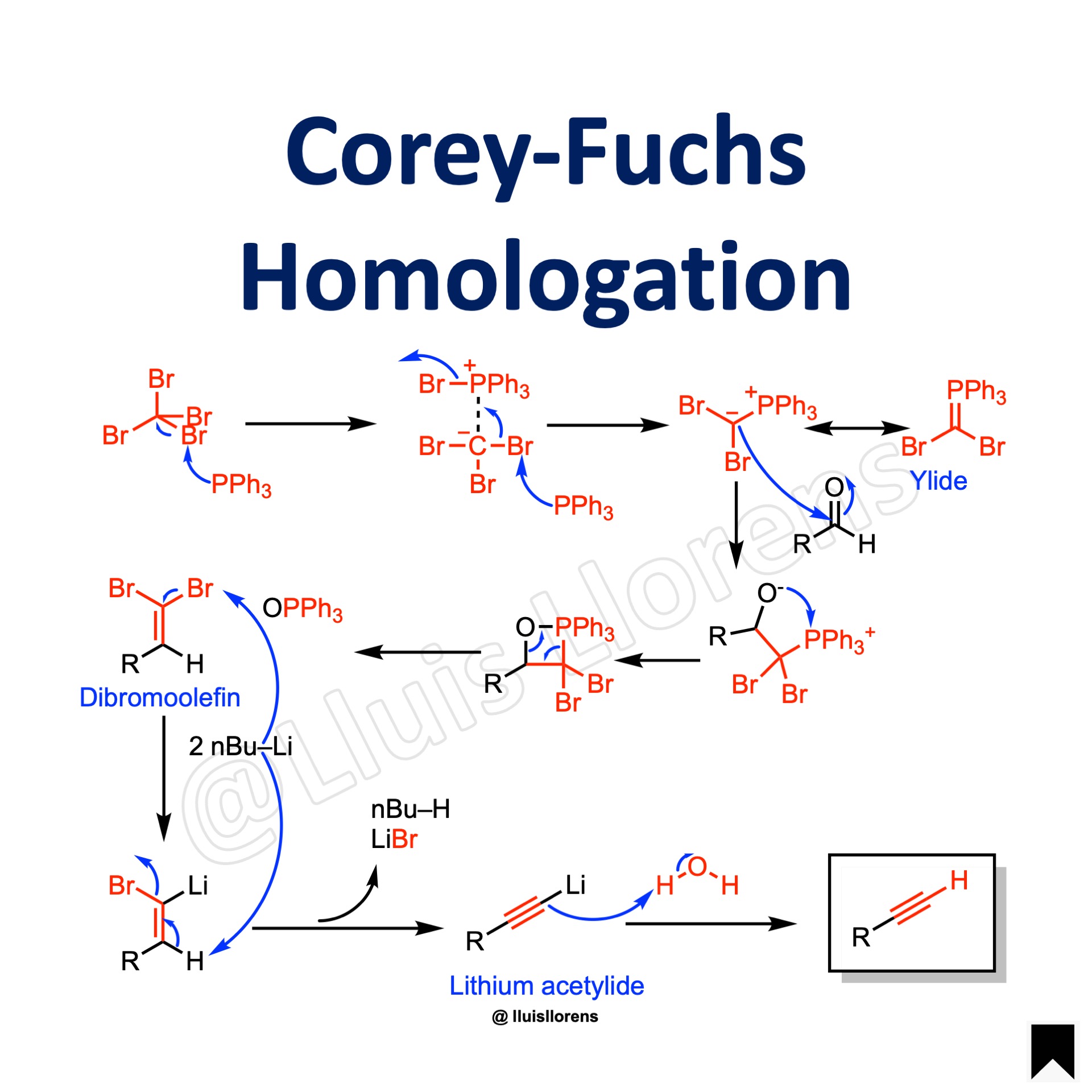
Reaction Mechanism
1. Generation of the phosphorous ylide. 2. Reaction of the ylide with the aldehyde delivers the homologated dibromoolefin in a Wittig-type step. 3. Final conversion of the dibromoolefin to a terminal alkyne is achieved through a lithium acetylide intermediate (lithium-halogen exchange and elimination).
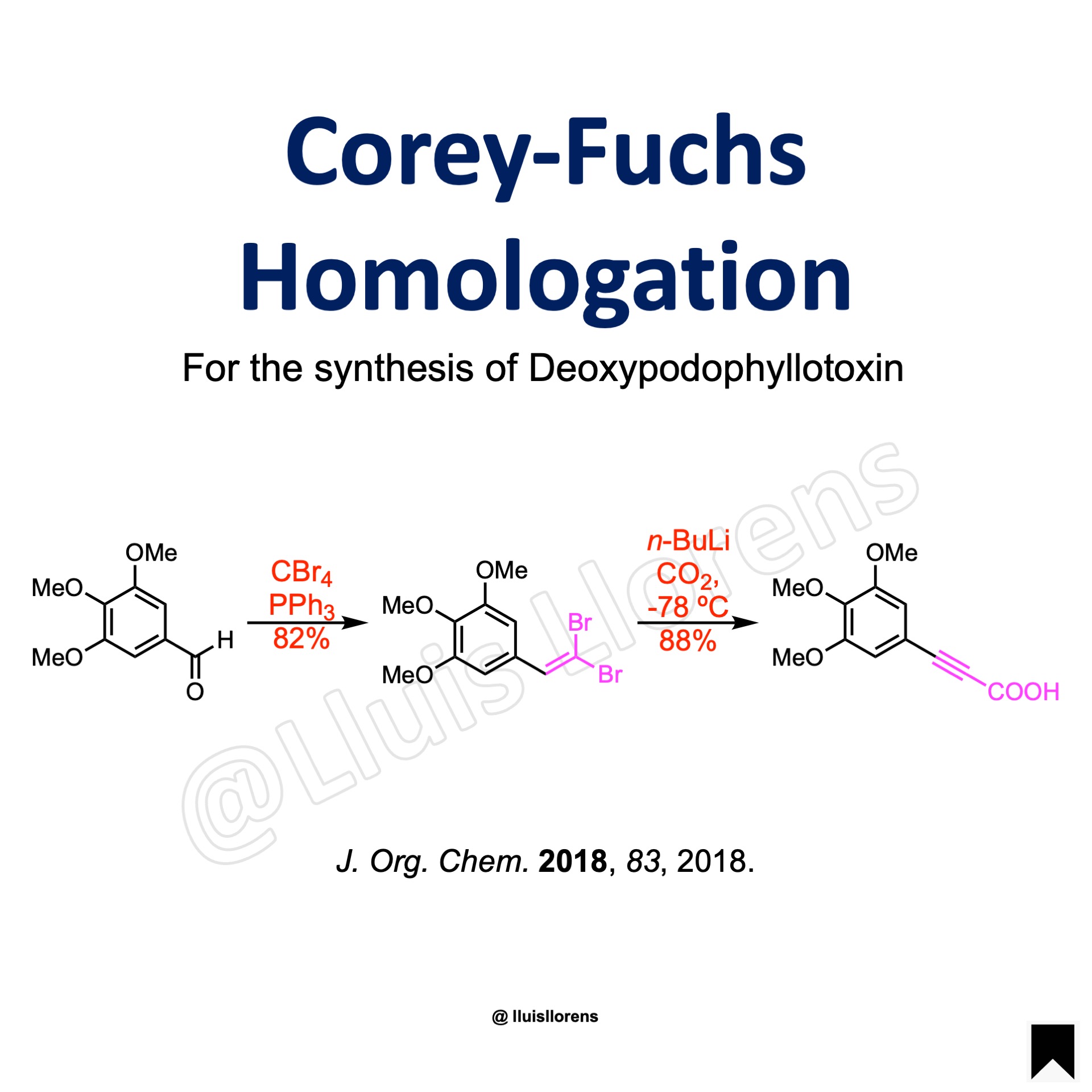
Example
Experimental Procedure
STEP 1: To a solution of triphenylphosphine (3.0 eq) in dry DCM (153 mL) cooled to 0 °C under argon was added carbon tetrabromide (1.5 eq), and the resulting mixture was stirred at 0 °C for 15 min. To the solution was added the benzaldehyde (15.29 mmol, 1.0 eq) in dry DCM (8 mL). The resulting mixture was stir at room temperature overnight. The mixture was triturated with cold hexanes and filtered to remove excess triphenylphosphine. The filtrate was concentrated and subjected to silica gel chromatography to obtain the dibromoolefin product (82% yield).
STEP 2: To a solution of the alkene (9.43 mmol, 1.0 eq) in dry THF (16.00 mL) under argon cooled to –78 °C (dry ice / acetone bath) was added a solution of n-butyllithium (2.5 M, 1.9 eq), and the solution was stirred for 20 min at –78 °C before solid carbon dioxide (crushed, 10.0 eq) was added at once. The mixture was gradually allowed to warm to room temperature before poured into water. A 1:1 mixture of EtOAc/ hexanes was added, and the aqueous part was acidified (pH 2) with HCl (6.0 N) and extracted with ethyl acetate. The organic layers were combined, dried (MgSO4) and concentrated to access the desired propiolic acid (88% yield).
Learn More Named Reactions